|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A decade ago the answer to these two questions was no. Today, as we indicated above, the answer is yes. Some operations incur such low rates of morbidity and mortality that a test is not indicated unless it is necessary for "routine" preventive care of the patient. Examples would be diagnostic knee arthroscopy and cataract extraction. Table 25-6 divides procedures into three types. Type A procedures are minimally invasive operations that produce little tissue trauma and minimal blood loss. I believe that no laboratory testing is indicated for these operations, based on preoperative status alone (such a division was proposed in the ASA Advisory for Preanesthesia Evaluation discussed earlier). That assumes that the patient is seen
What abnormalities on chest radiographs can influence management of anesthesia? Certainly it may be important to know about the existence of the following conditions before proceeding to anesthesia and surgery: tracheal deviation or compression; mediastinal masses; pulmonary nodules; a solitary lung mass; aortic aneurysm; pulmonary edema; pneumonia; atelectasis; new fractures of the vertebrae, ribs, and clavicles; dextrocardia; and cardiomegaly. However, a chest radiograph probably would not detect the degree of chronic lung disease requiring a change in anesthetic technique any better than would the history or physical examination.
Table 25-10 [112] [113] [149] [156] [166] [170] [176] [177] [178] [179] [180] [181] [182] [183] [184] [185] [186] [187] [188] [189] [190] [191] [192] [193] [194] [195] [196] [197] shows the prevalence of conditions that a chest radiograph might detect. These data show that abnormalities are rare in the asymptomatic individual. In fact, the risks associated with chest radiographs probably exceed their possible benefit if the patient is asymptomatic and less than 75 years of age. This analysis is predicated on maximizing benefit to all patients as a general group, because one cannot say which individual patients will benefit and which will be harmed. Thus, chest radiographs are not warranted for any asymptomatic patient who is less than 75 years of age and free of risk factors. In fact, this conclusion may apply to those older than 75 years of age as well.
The incidence of ECG abnormalities has been determined by studies of patients (Hsu J et al, unpublished data)[113] [114] [170] [171] [202] [203] [204] [205] [206] [207] [208] and epidemiologic surveys of healthy people. [183] The abnormalities on the ECG that have the potential to alter management of anesthesia are as follows: atrial flutter or fibrillation; first-, second-, and third-degree atrioventricular block; changes in ST segment suggesting myocardial ischemia or recent pulmonary embolism; premature ventricular and atrial contractions, especially those that are frequent (i.e., greater than 3 per minute); left or right ventricular hypertrophy; short PR interval; Wolff-Parkinson-White syndrome; myocardial infarction; prolonged QT segment (this has special importance and gained prominence as a cause of perioperative morbidity and mortality); and tall peaked T waves. What is the incidence of finding these abnormalities on the 12-lead preoperative screening ECG but not on a standard monitor lead 1 or an MCL5 lead applied immediately before induction of anesthesia in the OR?
Before answering that question, some qualifiers apply. First, few of the studies on the incidence of ECG abnormalities (Apfelbaum JL et al, unpublished data)[78] [79] [167] excluded patients having histories or physical examinations indicating cardiac problems. Second, the studies do not distinguish those findings evident on monitoring lead from findings evident on only 6- or 12-lead ECGs[112] [113] [114] [128] [136] [145] [146] [156] [170] [183] [185] [186] [187] [188] [189] [190] [191] [192] [193] [194] [195] [196] [197] [198] [199] [200] [201] [202] [203] [204] [205] [206] [207] [208] [209] [210] ( Table 25-12 ).
|
|
|
|
|
|
Specific Abnormalities | |||
|---|---|---|---|---|---|---|---|---|
| Age (y) | Sex | Series | Patients Examined (n) | Total Abnormalities † (%) | LVH | MI | ST Changes | AV Block |
| 16–19 | M | Ostrander et al[204] | 216 | 20.3 | 17.8 | 0.0 | 0.9 | 1.4 |
| 16–19 | F | Ostrander et al[204] | 24 | 5.9 | 1.3 | 0.0 | 4.2 | 0.4 |
| 20–29 | M | Ostrander et al[204] | 452 | 14.0 | 7.1 | 0.2 | 6.0 | 0.7 |
| 20–29 | F | Ostrander et al[204] | 577 | 11.3 | 0.2 | 0.2 | 9.9 | 1.0 |
| 20–29 | M | Collen et al[170] | 3,000 | 9.6 | — | — | — | — |
| 30–39 | F | Collen et al[170] | 4,000 ‡ | 9.3 | — | — | — | — |
| >30 | Either | Maigaard et al[183] | 1,256 | >4.5 | — | 0.1 | — | — |
| 30–39 | M | Ostrander et al[204] | 676 | — | 3.0 | 0.0 | 6.9 | 1.3 |
| 30–39 | F | Ostrander et al[204] | 699 | — | 0.4 | 0.1 | 11.6 | 1.6 |
| 30–39 | M | Collen et al[170] | 4,000 ‡ | 12.1 | — | — | — | — |
| 30–39 | F | Collen et al[170] | 5,000 ‡ | 11.7 | — | — | — | — |
| 35–44 | M | Kannel et al[203] [205] | — | — | 2.9 | — | — | — |
| 35–44 | F | Kannel et al[203] [205] | — | — | 0.9 | — | — | — |
| <40 | Either | Blery et al[128] | 2,256 | 0.6 | — | — | — | — |
| <40 | Either | Apfelbaum et al[206] , § , ‖ | 510 | 0 | — | — | — | — |
| <40 | Either | McKee and Scott[112] , § | 23 | 13 (0) | — | — | — | — |
| <40 | Either | Gold et al[209] | 94 | 37 | — | — | — | — |
| <40 | Either | Velanovich[185] | NA | 15.8 | — | — | — | — |
| >40 | Either | Johnson et al[114] | 212 | 66 | 2.8 | 13.2 | 23.1 | 2.8 |
| 40–49 | M | Ostrander et al[204] | 468 | 24 * | 4.1 | 1.7 | 16.1 | 1.5 |
| 40–49 | F | Ostrander et al[204] | 474 | 21 ‡ | 0.6 | 0.8 | 17.2 | 0.6 |
| 40–49 | M | Collen et al[170] | 4,000 ‡ | 17.6 | — | — | — | — |
| 40–49 | F | Collen et al[170] | 5,000 ‡ | 15.6 | — | — | — | — |
| 40–49 | Either | Gold et al[209] | 260 | 36 | — | — | — | — |
| 41–50 | Either | McKee and Scott[112] , § | 53 | 1.9 (0) | — | — | — | — |
| <45 | ? | Moorman et al[207] , § | 275 | 0.4 | — | — | — | — |
| 45–54 | M | Kannel et al[203] [205] | — | — | 4.8 | — | — | — |
| 45–54 | F | Kannel et al[203] [205] | — | — | 3.6 | — | — | — |
| 40–60 | Either | Velanovich[185] | NA | 47.5 | — | — | — | — |
| >45 | ? | Morman et al[207] , § | 500 | 1.4 | — | — | — | — |
| >50 | Either | Yipintsoi et al[202] | 424 § | 14.4 § | — | — | — | — |
| 50–59 | M | Ostrander et al[204] | 330 | 30 ‡ | 3.3 | 5.1 | 20.8 | 1.2 |
| 50–59 | F | Ostrander et al[204] | 327 | 40 ‡ | 3.4 | 0.9 | 32.4 | 2.1 |
| 50–59 | M | Collen et al[170] | 5,000 ‡ | 24.9 | — | — | — | — |
| 50–59 | F | Collen et al[170] | 6,000 ‡ | 20.7 | — | — | — | — |
| 50–59 | Either | Gold et al[209] | 163 | 44 | — | — | — | — |
| 51–60 | Either | McKee and Scott[112] , § | 84 | 23.8 (0) | — | — | — | — |
| 55–64 | M | Kannel et al[203] [205] | — | — | 10.1 | — | — | — |
| 55–64 | F | Kannel et al[203] [205] | — | — | 4.1 | — | — | — |
| <60 | Either | Rabkin and Horne[145] [146] | 309 | 13.5 | 2.5 | 1.6 | 11.0 | 1.0 |
| >60 | Either | Rabkin and Horne[145] [146] | 503 | 24.4 | 2.2 | 1.9 | 13.0 | 0.6 |
|
|
|
|
— | 42.3 | — | — | — | — |
| >60 | Either | McKee and Scott[112] , § | 163 | (1.3) | — | — | — | — |
| >60 | Either | Gold et al[209] | 134 | 62 | — | — | — | — |
| >60 | Either | Velanovich[185] | — | 61.5 | — | — | — | — |
| >65 | Either | Seymour et al[208] | 222 | 52.7 |
|
|
|
|
| 60–69 | M | Ostrander et al[204] | 177 | — | 8.4 | 9.0 | 37.1 | 4.5 |
| 60–69 | F | Ostrander et al[204] | 196 | — | 10.2 | 6.1 | 42.4 | 4.1 |
| 60–69 | M | Collen et al[170] | 2,000 ‡ | 35.1 | — | — | — | — |
| 60–69 | F | Collen et al[170] | 3,000 ‡ | 29.7 | — | — | — | — |
| 65–74 | M | Kannel et al[203] [205] | — | — | 7.1 | — | — | — |
| 65–74 | F | Kannel et al[203] [205] | — | — | 9.6 | — | — | — |
| >?70 | Either | Wolf-Klein et al[137] | 500 | 11.9 | — | — | — | — |
| >70 | M | Collen et al[170] | 1,000 ‡ | 52.2 | — | — | — | — |
| >70 | F | Collen et al[170] | 1,000 ‡ | 41.2 | — | — | — | — |
| ≥71 | Either | Levinstein et al[156] | 121 | 24.4 | 0.7 | 13.8 | 6.3 | 3.6 |
| 70–79 | M | Ostrander et al[204] | 100 | — | 7.9 | 9.9 | 46.5 | 7.9 |
| 70–79 | F | Ostrander et al[204] | 119 | — | 11.8 | 2.5 | 43.8 | 6.7 |
| 74–97 | Either | Domoto et al[136] | 69 | 27.5 | — | — | — | — |
| >80 | M | Ostrander et al[204] | 26 | — | 11.5 | 7.7 | 46.2 | 19.2 |
| >80 | F | Ostrander et al[204] | 43 | — | 16.3 | 4.7 | 58.2 | 9.3 |
| 0–?90 | Either | Blery et al[128] , § | 2,256 | 0.6 (0.3) | — | — | — | — |
| 0–?90 | Either | Turnbull and Buck[113] | 632 | 14.6 | Combined: 0.67 | |||
| 0–?90 | Either | Muskett and McGreevy[115] | 145 | 1.3 | — | — | — | — |
| AV, atrioventricular; LVH, left ventricular hypertrophy; MI, myocardial infarction. | ||||||||
|
|
New Abnormality with a previously | |||
|---|---|---|---|---|
|
|
Normal ECG | Abnormal ECG | ||
| Age(y) | <60 | ≥60 | <60 | ≥60 |
| No. of patients/total no. | 18/180 | 42/192 | 24/129 | 81/310 |
| % new abnormalities | 10 | 21.9 | 18.6 | 26 |
| Abnormality |
|
|
|
|
| T wave | 11 (6.1%) | 18 (9.4%) | 10 (7.8%) | 19 (6.1%) |
| ST segment | 7 (3.9%) | 9 (4.7%) | 6 (4.7%) | 20 (6.4%) |
| Arrhythmias |
|
|
|
|
| SVT or PVC | 3 (1.7%) | 7 (3.6%) |
|
8 (2.6%) |
| Others, including PAC | 3 (1.7%) | 6 (3.1%) | 1 (0.8%) | 1 (0.3%) |
| QRS duration |
|
8 (4.2%) | 2 (1.6%) | 14 (4.5%) |
| LVH | 3 (1.7%) | 4 (2.1%) | 5 (3.9%) | 7 (2.3%) |
| Q wave | 4 (2.2%) | 3 (1.6%) | 1 (0.8%) | 7 (2.3%) |
| Ventricular conduction defects | 5 (2.6%) | 1 (0.8%) | 7 (2.3%) | 7 (2.3%) |
| AV block | 2 (1.0%) | 3 (2.3%) | 1 (0.3%) |
|
| AV, atrioventricular; ECG, electrocardiogram; LVH, Left ventricular hypertrophy; PAC, premature atrial contractions; PVC, premature ventricular contractions; SVT, supraventricular tachycardia. | ||||
| Data from Rabkin and Horne.[145] [146] | ||||
The data in Table 25-13 and elsewhere[210] [211] show that abnormalities on the ECG are relatively common and increase exponentially with age. Averaging all those data indicates that the incidence of abnormal preoperative electrocardiographic results will exceed 10% at 40 years of age and will be 25% by 60 years of age. These estimates pool abnormalities for both sexes. Clearly, the studies that looked for abnormalities on the ECG after first ensuring that the patient was asymptomatic[112] [202] [128] found a much lower incidence of significant abnormalities. McKee and Scott[112] found no abnormalities significant to perioperative care for 160 individuals who had no cardiac symptoms and were under 60 years of age, and only two abnormalities for 163 patients over 60 years of age. Moorman and colleagues[207] reported that only 1 of 275 asymptomatic patients 45 years of age or less had abnormalities on preoperative ECGs. In the study by Blery and co-workers,[128] only 0.6% of 2,256 patients under age 40 who had no cardiac or pulmonary symptoms had an abnormality on preoperative ECGs. Our group found no abnormalities on ECG that were significant to, or altered, perioperative care for 510 patients judged to be asymptomatic on the basis of results from a video questionnaire[206] (Apfelbaum JL et al, unpublished data). This study is discussed later in this chapter.
How useful is it to repeat ECGs if the patient has had an ECG within the past 2 years? Rabkin and Horne[145] [146] addressed this question. "New abnormalities" on a subsequent ECG occur with significant frequency—approximately 25% to 50% as frequently as all abnormalities occurring on the previous ECG (see Table 25-13 ). Thus, one would be justified in obtaining screening ECGs prior to elective surgery for all patients over 40 years of age, even those who have recently had an ECG if it is more than 5 months old or was abnormal. (See Table 25-11 for an analysis of the risk versus benefit of obtaining an ECG for asymptomatic patients.)
Some physicians have questioned even that conclusion. Goldberger and O'Konski[176] believe that the most important potential benefit of the preoperative ECG is detection of previously unrecognized myocardial infarction. This risk increases with age. However, even for the highest-risk group, men 75 years of age or older, the estimated incidence of unrecognized Q-wave infarction within the preceding 6 months is relatively small (< 0.5%). Goldberger and O'Konski concluded that the risk from obtaining a preoperative ECG and subsequent reactions probably exceeds its benefit if patients are asymptomatic, do not have important risk factors for coronary disease, and are under 45 (men) or 55 (women) years of age. If we use the data in Table 25-12 and apply the benefit-risk analysis described in the section on chest radiographs, ECGs are indicated for asymptomatic patients of average risk (no special family history of premature arterial aging diseases such as stroke, heart attack, or peripheral vascular disease prior to age 40) undergoing type B or C procedures who are over 40 (men) or 50 (women) years of age.
These estimates do not account for the differences in physiologic age that exist between patients of the same calendar age. I believe that these differences are important. Although I also believe that physiologic age ("RealAge" * ) should be the factor used to determine the need for testing, this hypothesis has not been widely tested. We know that conditions such as normal blood pressure, regular vigorous exercise, the absence of exposure
The classic references on hemoglobin, hematocrit, and white blood cell counts, and the inferences they allow, have stood the test of time. We describe them now, so that the reader will understand the rationale for decisions and practices used today.
In 1964, Wasserman and Gilbert[214] reported that of 28 patients with uncontrolled polycythemia (hemoglobin >16 g/dL) who underwent major surgery, 22 (79%) had complications and 10 (36%) died. This group was compared with 53 patients who had controlled polycythemia (hemoglobin <16 g/dL) and major surgery; 15 (28%) had complications, and 3 (5%) died. In both groups, most of the complications were related to polycythemia (e.g., hemorrhage or thrombosis). Other data confirm that polycythemia is an independent risk factor for cardiovascular mortality.[215] Admittedly, the study of Wasserman and Gilbert had deficiencies: it was a retrospective study, no time frame was given, and "minor" surgery was excluded. Also, the study did not explain why polycythemia was controlled preoperatively for some patients but not for others. Nevertheless, knowledge and pretreatment of polycythemia decreased perioperative morbidity and mortality, but there is little or no data on what levels are appropriate to treat. Most physicians arbitrarily rely on the limited data of Wasserman and Gilbert[214] and do not treat unless the hemoglobin level is greater than 16 mg/dL.
Not even such weak evidence exists for treatment of normovolemic anemia. Rothstein[216] concluded that hemoglobin of 9 g/dL is adequate for patients over 3 months of age but should exceed 10 g/dL for younger patients (see Chapter 47 and Chapter 48 ). As emphasized by Roy and colleagues,[118] the age of the patient at the time of discovery of anemia often points to its cause. Anemia in the neonatal period is often attributable to recent blood loss, isoimmunization, congenital hemolytic anemia, or congenital infection. Anemia first detected 3 to 6 months after birth suggests a congenital disorder of hemoglobin synthesis or structure. Therefore, assays of hemoglobin concentration in the first 6 months may represent the first opportunity for analysis. This use of the perioperative period for screening (or case finding) necessitates a proactive procedure for referring the patient and parents for counseling and/or treatment (ideally integrated through an automated information [informatics] system linking preoperative clinic and primary care physicians). Thus, preoperative evaluation can add to a patient's prior medical database.
This point—that preoperative evaluations and documentation of data from the history are often not complete—was reiterated by Hackmann and colleagues[117] in their study of the prevalence of anemia. They reported that several patients whose anemia was not properly suspected had conditions (Hirschsprung's disease, pyloric stenosis, or history of anemia accompanying juvenile rheumatoid arthritis) that should have alerted the anesthesiologist. Similarly, it was difficult to find a perioperative benefit in search for even common hemoglobinopathies (see later) in asymptomatic individuals.[217] These investigators also emphasized the importance of obtaining a thorough history.
Should asymptomatic anemia be treated prior to non-blood-loss surgery, as O'Connor and Drasner[116] and others have done? Does this practice produce more benefit than harm? Does iron therapy at this early age contribute to late ischemic heart disease? Is this the function of preoperative testing—to spot chronic conditions? Although there are no definite answers to these questions, I agree with O'Connor and Drasner that these practices are indicated if one sets up the preoperative assessment to function in that role. This means that the anesthesiologist and the clinic would constitute second-opinion consultants to the primary care physician. It would also require that the preoperative assessment be performed sufficiently early (at least 1 week before surgery) and be vested with enough authority to postpone surgery in a timely enough fashion to avoid any decrease in OR efficiency.
If the preoperative assessment is not done in advance or is not vested with sufficient authority, perhaps asymptomatic anemia prior to non-blood-loss surgery should not be treated preoperatively. This seems to be confirmed by studies showing that patients survive anesthesia and type-A surgery when hemoglobin levels are above 8.0 g/dL.[218] [219] No data confirm the hypothesis that preoperative treatment of moderate or mild normovolemic anemia in such patients decreases perioperative morbidity or mortality. Similarly, no data exist regarding the possible harm from abnormal white blood cell counts found preoperatively. Therefore, the following ranges of "surgically acceptable values" are arbitrary: for hematocrit, 29% to 57% for men and 27% to 54% for women; for white blood cell count, 2,400 to 16,000/mm3 for both men and women. When values fall outside these ranges, I recommend seeking an alternative diagnosis before instituting anesthesia or surgery.[219]
How many healthy patients have this degree of abnormality in hematocrit or white blood cell count? No such patient was found in either study of the 223 and 2,010 patients judged healthy by history (i.e., history indicated no need for tests).[111] [206] Table 25-14 provides the only other available data, which are limited. If we assume that 10% of all abnormalities are outside the "surgically acceptable" range (see Table 25-14 ), and if we apply the benefit-risk analysis described in the section on chest radiographs, we would conclude that either preoperative hematocrit or hemoglobin levels should be determined for all female surgical patients and for all male surgical patients over 64 years of age who are undergoing type B or C surgical procedures. Red cell antigen screening would be warranted for all patients undergoing procedures involving possible blood loss of more than 2 U/70 kg body weight (type B and C surgical procedures).[221] [222] White blood cell counts appear to be rarely, if ever, justified for asymptomatic patients.
| Age (y) | Sex | Series | Patients Examined (n) | Hemoglobin Abnormalities (%) | WBC Count Abnormalities (%) |
|---|---|---|---|---|---|
| <1 | Either | Roy et al[118] | 257 | 1.1 |
|
| <1 | Either | Hackmann et al[117] | 269 | 2.6 |
|
| 1–5 | Either | Roy et al[118] | 1,113 | 0.7 |
|
| 3–12 | Either | Nigam et al[119] | 250 | 0.8 |
|
| 5–18 | Either | Roy et al[118] | 630 | 0 |
|
| <18 | Either | Hackmann et al[117] | 2,649 | 0.5 |
|
| <18 | Either | Baron et al[120] | 1,863 | 1.1 |
|
| <18 | Either | O'Connor and Drasner[116] | 468 | 11.9 | 2.7 |
| 1–19 | Either | Hackmann et al[117] | 2,380 | 0.3 |
|
| <19 | Either | Wood and Hoekelman[143] | 1,924 | 0.8 |
|
| <40 | M | Collen et al[170] | 6,941 | 1.9 | 2.6 |
| <40 | F | Collen et al[170] | 9,037 | 12.6 | 2.6 |
| ≥18 | Either | Parkerson[159] | 392 | 18.8 | 10.7 |
| >19 | Either | Johnson et al[114] | 212 | 2.4 * | 0 |
| 32–90 | Either | Johnson and Mortimer[124] | 100 * | 12 | 7 |
| <40 | Either | McKee and Scott[112] , * | 96 * | 0 | 0 |
| <40 | Either | Velanovich[185] | — † | 12 |
|
| 40–59 | M | Collen et al[170] | 11,832 | 3.1 | 2.2 |
| 40–59 | F | Collen et al[170] | 9,657 | 10.1 | 2.2 |
| 41–50 | Either | McKee and Scott[112] , * | 53 * | 5.7 | ? |
| 40–60 | Either | Velanovich[185] | — † | 15.5 |
|
| 51–60 | Either | McKee and Scott[112] , * | 85 * | 0 | 0 |
| >-60 | M | Collen et al[170] | 4,062 | 5.6 | 1.7 |
| ≥60 | F | Collen et al[170] | 3,134 | 5.5 | 1.7 |
| >60 | Either | McKee and Scott[112] , * | 163 * | 6.1 | ? |
| >60 | Either | Velanovich[185] | — † | 31.6 |
|
| >?70 | Either | Wolf-Klein et al[137] | 551 | 33.2 | 17.4 |
| >71 | Either | Levinstein et al[156] | 121 | 27.8 | 4.0 |
| 74–97 | Either | Domoto et al[136] | 70 | 11.4 | 4.3 |
| Unspecified | Either | Turnbull and Buck[113] | 1,005 * | 0.7 | 0.1 |
| Unspecified | Either | Muskett and McGreevy[115] | 199 | 60.8 | 34.8 |
|
|
|
|
|
9.0 ‡ | 5.1 ‡ |
| Unspecified | Either | Kaplan et al[111] , * | 293 | 0 ‡ | 0 ‡ |
| Unspecified | Either | Gold and Wolfersberger[223] , * | 3,375 | 0.33 |
|
| Unspecified | Either | Carmalt et al[220] | 278 | 30.4 § |
|
| Unspecified | Either | Huntley et al[161] | 119 | 23 |
|
| Unspecified | Either | Williamson et al[160] | 982 | 3.2 |
|
| Unspecified | Either | Blery et al[128] , * | 1,728 | 1.1 |
|
What blood chemistries would have to be abnormal, and how abnormal would they have to be, to justify changing one's perioperative management? Abnormal hepatic or renal function might change the choice and dose of anesthetic or adjuvant drugs. Approximately 1 in 700 supposedly healthy patients actually harbors hepatitis, and 1 in 3 of those patients will become jaundiced.[224] [225] However, our group found no asymptomatic patient who denied exposure to hepatitis who then became jaundiced after uneventful surgery [206] (Roizen MF, unpublished data for more than 11,500 patients in a prospective study of the "HealthQuiz" [discussed later]; Hsu J et al, unpublished data). These data suggest that either the screening history suffices or the incidence of asymptomatic hepatitis is decreasing.
Data from the National Veterans Affairs Surgical Risk Study[61] found that the albumin level was an important predictor of perioperative morbidity and mortality in every surgical specialty. Therefore, one could argue for
Table 25-15 * presents the available data regarding abnormalities found on screening blood chemistries. Unexpected abnormalities are reported for 2% to 10% of patients screened, and these abnormalities lead to many additional tests that usually (approximately 80%) have no significance for the patient. In fact, as described in the section on laboratory tests as ineffective screening devices, if 20 chemistry tests are ordered for a healthy individual, there is a 64% chance that results of at least one test will be abnormal (see Fig. 25-3 ). Unexpected abnormalities that are significant arise in 2% to 5% percent of patients studied. Of these abnormalities, approximately 70% percent are related to blood glucose[226] and blood urea nitrogen (BUN) levels. Note that the screen for diabetes may soon shift from random determinations of blood glucose levels to determination of the concentration of glycosylated hemoglobin (Hb A1c ). The 9 to 20 additional tests on the screening simultaneous multi-channel analysis (SMA) of 12 to 20 variables lead to very few important discoveries affecting anesthesia. In fact, the false-positive rate is so high (i.e., 96.5% for the test for calcium) that the value representing cost versus benefit for most of these tests (even when the tests are free) is negative, as is the value representing benefit versus risk. Examination of the data reported by Berwick[168] in Table 25-15 helps clarify the difficulty of screening. More than 75% of the abnormalities (see the line in Table 25-15 in which n = 76,519) were not even outside the range that caused the laboratories at these health screening fairs to notify the patient or physician of an abnormal value (see the line in Table 25-15 in which n = 86,006), let alone be judged significant to the patient's health.
It appears that only blood glucose (or its successor Hb A1c ), albumin, and BUN assays are indicated before type B and C procedures. Even then, BUN is indicated only for patients over 65 years of age, and glucose (or Hb A1c ) only for individuals over 75 years of age. No blood chemistry tests are warranted for patients less than 65 years of age. Furthermore, because the antibody test for hepatitis A and C are useful after infection has occurred, the medicolegal risk posed by postanesthetic jaundice should be even less.
Abnormalities are common on urinalysis † ( Table 25-16 ) (Apfelbaum JL et al, unpublished data). The quality of urinalysis results obtained by dipstick technique has been variable at best.[236] In addition, these abnormal results usually do not lead to beneficial changes in management. Most of the results that do lead to beneficial changes could have been obtained by history or determination of BUN and glucose (or Hb A1c ) levels, tests that are already recommended for all patients over 65 and 75 years of age, respectively. Thus, urinalysis, although initially inexpensive, becomes an expensive test to justify on a benefit-cost or benefit-risk basis.
Although partial thromboplastin time (PTT) and prothrombin time (PT) are useful tests for screening patients who have a history of bleeding, their value as screening tests for asymptomatic patients has never been shown * ( Table 25-17 ). Virtually no asymptomatic patient in the literature has had unequivocal benefit from clotting function studies performed preoperatively (see Table 25-17 ). Most patients show symptoms or have a medication history suggesting the possible need for clotting function tests. Suchman and Mushlin[242] and Macpherson[243] reviewed the data, as we did, [82] and came to the same conclusion: preoperative clotting function testing for asymptomatic patients who have no risk factors for coagulopathy is incapable of predicting perioperative bleeding. No information is gained from either an abnormal or a normal result on clotting studies in low-risk patients. Figure 25-5 presents an algorithm that can be used to segregate high- and low-risk patients.[244]
One patient deserves special comment: the patient taking aspirin or aspirin-containing compounds. Aspirin at a dose of 3 to 10 mg/kg of body weight daily does not seem to pose a risk of bleeding, and is even beneficial enough for some groups to begin instituting it in preoperative clinics.[43] [44] But this practice is just now going from banned to advocated due to early evidence of benefit to patient outcome.
Because the pharmacokinetics of aspirin change when more than 2 g/70 kg of body weight is consumed daily, a patient should be evaluated if he or she is on large doses of aspirin (more than six 325-mg tablets a day) and has not discontinued aspirin consumption sufficiently early to ensure that there is no appreciable level of acetylsalicyclic acid in the blood for 24 hours before surgery. (This is the period in which acetylsalicylic acid would have to be absent for the generation of the approximately 50,000 new platelets/mm3 needed for normal platelet aggregation.) The patient should also be evaluated if surgical hemostasis cannot be ensured or if a regional procedure into a closed space is planned. Nonsteroidal anti-inflammatory drugs (NSAIDs) present similar problems.[245] (For further explanation of these situations, see the section in Chapter 27 regarding interruption of a drug regimen prior to surgery.)
Tests for HIV infection and pregnancy and screening for hemoglobinopathy and malignant hyperthermia raise ethical questions that may require close attention to institutional policy and the immediate availability of counseling services. Moreover, all of these tests have risks. The physician may therefore decide to limit testing to only "at-risk" populations (e.g., for pregnancy testing, only female patients who believe they may be pregnant). As noted previously, the ASA Practice Advisory survey of members found that 17% of all members obtained a
| Age (y) | Series | Patients Examined (n) | BUN | Cr | Glucose | AST or SGOT | Uric Acid | Cholesterol | Albumin | Total Protein | Ca2+ | VDRL | ALK PTAse | Bilirubin | K+ | Phosphate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–65 | Narr et al[110] | 3,782 | — | — | 1.9 | 0.3 | — | — | — | — | — | — | — | — | 0.2 | — |
| 10–54 | Schemel[225] | 7,620 | — | — | — | 0.144 | — | — | — | — | — | — | — | — | — | — |
| 12–90 | Alsumait et al[125] | 1000 | — | 1.6 | 8.2 | — |
|
— | — | — | — | — | — | — | 4.0 | — |
| 15–85 | Carmalt et al[220] , * | 296 | 1.4 | 1.0 | 2.0 | 0 | — | 0.3 | 0 | — | 0.3 | — | 0 | 0 | 0.3 | — |
| >18 | Parkerson[159] | 397 | — | 1.2 | 15.8 | 2.8 | 7.9 | 6.1 | — | — | 2.0 | — | — | 1.2 | 6.6 | — |
| >18 | Schneiderman et al[227] | 547 | — | 9.3 | — | 1.3 | — | — | — | — | — | — | 9.7 | 3.7 | — | — |
| >18 | [Bryan data][228] [229] | 623 | 1.1 | — | 5.0 | — | 4.5 | — | 1.4 | 2.4 | 1.0 | — | — | — | 4.0 | — |
| >25 | Peery[230] , * | 1,771 | 18 | — | 21 | 3.1 | 36 | 30 | 0.5 | 0.5 | — | — | 1.3 | — | 3.6 | — |
| 32–90 | Johnson and Mortimer[124] | 100 | 17 | 14 | 8 | — | — | — | — | — | — | — | — | — | 6 | — |
| <40 | McKee and Scott[112] , † | 96 | 0 | — | — | — | — | — | — | — | — | — | — | — | 0 | — |
| 40–59 | Collen et al[170] | 21,489 | — | 1.4 | 5.6 | 4.6 | 4.8 | 2.7 | 0.3 | 4.4 | 1.3 | 1.9 | — | — | — | — |
| 41–50 | McKee and Scott[112] , † | 53 | 0 | — | — | — | — | — | — | — | — | — | — | — | 0 | — |
| >40 | Collen et al[170] | 15,978 | — | 0.8 | 4.6 | 3.6 | 3.4 | 1.7 | 0.4 | 3.5 | 1.4 | 0.8 | — | — | — | — |
| 51–60 | McKee and Scott[112] , † | 85 | 0 | — | — | — | — | — | — | — | — | — | — | — | 0 | — |
| >60 | Collen et al[170] | 7,196 | — | 2.7 | 8.3 | 4.5 | 6.0 | 3.0 | 0.4 | 3.9 | 1.5 | 2.3 | — | — | — | — |
| >60 | McKee and Scott[112] , † | 163 | 2.5 | — | — | — | — | — | — | — | — | — | — | — | 0 | — |
| >?70 | Wolf-Klein et al[137] | 500 | 24.6 | 14.5 | 24.6 | 9.4 | 7.7 | 13.8 | 20.4 | 9.9 | 7.2 | — | 23.2 | 1.7 | 5.4 | — |
| ≧71 | Levinstein et al[156] | 121 | 36 | 10.8 | 29.0 | 0.8 | 7.2 | 6.9 | 27.8 | 19.2 | 5.6 | — | 19.0 | 0.7 | 3.0 | — |
| 74–97 | Domoto et al[136] | 70 | 30 | — | 26.5 | 9.2 | 8.6 | 17.2 | 0 | 2.1 | 1.0 | — | 9.2 | 2.1 | — | 1.0 |
| All | Wataneeyawech and Kelly[224] | 6,540 | — | — | — | 0.234 | — | — | — | — | — | — | — | — | — | — |
| All | Bryan et al[228] | 2,846 | 1.4 | — | 5.6 | — | — | — | 1.4 | 0.7 | 0.3 | — | — | — | 0.3 | — |
| All | Friedman et al[231] | 8,446 | 3.4 | 3.3 | 5.9 | 2.7 | 2.7 | 3.8 | 1.5 | 2.5 | 5.4 | — | 3.9 | 2.4 | 1.4 | — |
| All | Delahunt and Turnbull[139] , † | 332 | 0 | 0 | — | — | — | — | — | — | — | — | — | — | 0.3 | — |
| All | Young and Drake[233] , * | 390 | 6.4 | 3.7 | 7.5 | — | — | — | — | — | 2.0 | — | — | — | 0 | — |
| All | Berwick[168] | 86,006 ‡ | 0.7 | 0.3 | 1.2 | 0.5 | 1.2 | 0.8 | 0.02 | 0.09 | 0.5 | — | 0.7 | 0.3 | 1.8 | — |
| All | Berwick[168] | 76,519 | 1.6 | 1.3 | 3.5 | 2.5 | 2.9 | 5.1 | 1.5 | 1.2 | 2.8 | — | 2.6 | 4.0 | 3.2 | — |
| All | Boonstra and Jackson[233] , * | 12,000 | — | — | — | — | — | — | — | — | 5.0 * | — | — | — | — | — |
| All | Apfelbaum et al[206] , § | 1,784 ‖ | 2.0 | 1.8 | 3.4 | 1.8 | — | — | 0.3 | 1.3 | 1.0 | — | 1.3 | — | 2.6 ¶ | 0.3 |
| All | Apfelbaum et al[206] , § | Variable (asymptom.) ‡ | 0.3 | 0.3 | 0.1 | 0.2 | — | — | 0.2 | 0.3 | 0.7 | — | 0.6 | — | 1.8 ¶ | — |
| All | Whitehead[234] | 2,871 | 3.4 | — | 10.0 | 1.8 | 9.2 | 9.3 | 2.9 | — | 9.2 | — | 8.3 | 6.0 | 4.7 | — |
| All | Turnbull and Buck[113] , † | 995 | 0.1 | 0.2 | 0.7 | — | — | — | — | — | — | — | — | — | 1.4 | — |
| All | Blery et al[128] , † | ∼2,800 | 0.1 | 0.1 | 0.1 | — | — | — | — | — | — | — | — | — | 0.5 | — |
| ALK PTAse, alkaline phosphatase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca2+ , calcium; Cr, creatinine; K+ , potassium; SGOT, serum glutamicoxaloacetic transminase; VDRL, serologic test for syphilis, developed by the Venereal Disease Research Laboratory. | ||||||||||||||||
| Age (y) | Sex | Series | Patients Examined (n) | Abnormalities (%) | Significant Abnormalities (%) |
|---|---|---|---|---|---|
| <18 | Either | O'Connor and Drasner[116] | 453 | 15 | 0.4 |
| <19 | Either | Wood and Hoekelman[142] | 1,859 | 11.7 | 0.5 |
| Unspecified | Either | Huntley et al[161] | 119 | 61 | — |
| 5–12 | F | Cardiff-Oxford[235] | 16,800 | 1.8 | — |
| 15–? | Either | Lawrence and Kroenke[122] | 180 | 15 | <1.6 |
| Unspecified | Either | Gold and Wolfersberger[223] | 3,375 | 2.7 | — |
| Unspecified | Either | Williamson et al[160] | 982 | 16.7 | — |
| Unspecified | Either | Collen et al[170] | 44,663 | 14.6 | — |
| Unspecified | Either | Muskett and McGreevy[115] | 144 | 22.4 | — |
| Unspecified | Either | Berwick[168] | 235 | 2.6 | — |
| Unspecified | Either | Turnbull and Buck[113] , * | 995 | 4.3 | — |
| >19 | Either | Johnson et al[114] | 212 | 39 | <1.9 (?0) |
| >?70 | Either | Wolf-Klein et al[137] | 550 | 12.9 | — |
| ≥71 | Either | Levinstein et al[156] | 121 | 25.8 † | — |
| 74–97 | Either | Domoto et al[136] | 70 | 9.2 | — |
Testing of asymptomatic patients for AIDS is unlikely to be the most effective way of uncovering the disease. One program screening for HIV in asymptomatic individuals was able to produce an "acceptably low false-positive rate" by diagnosing HIV infection after only one sample of blood produced positive results on three different tests, and after the second sample of blood had been used for verification.[130] Thus, for pregnancy, hemoglobinopathies, and HIV infection, the history is still the best tool for identifying those who should be tested or those who are at risk of the condition.
In the past, no screening test has existed for susceptibility to malignant hyperthermia syndrome (MHS) other than a personal or family history of the condition (also see Chapter 27 ). Several new tests are available; none uses genetic testing, but one is expected to be available shortly. Despite previous studies suggesting a single localization of this disorder to chromosome 19q, Levitt and colleagues[246] and other groups have observed evidence for significant genetic heterogeneity in MHS. Nevertheless, they found seven MHS families in which the identified genetic aberrations appear linked to chromosome 17q11.2-24. Because the gene encoding the adult muscle sodium channel a-subunit (SCN4A) has also been localized to this region of the long arm of chromosome 17, this area may be the site of a primary defect in MHS. However, because the specific site cannot yet be identified, linkage analysis for MHS coupled with polymerase chain amplification might be used in the future to screen for MHS in high-risk patients.
It is still too early to predict the usefulness of these tests as a screening procedure for MHS or for other genetic diseases such as diabetes. Other screening tests for MHS have not been reliable enough to use for any but "at-risk" individuals (e.g., children with a history of myopathies who are undergoing surgery for strabismus, patients having a family history of problems with anesthesia, and patients with a history of abnormal (red) appearance, thermoregulation, and reactions to minor stresses) ( Fig. 25-6 ) (also see Chapter 27 ).
Magnesium (Mg) deficiency represents another special situation. Putatively it is much more common than other ion deficiencies, and Mg treatment has been advocated as extremely beneficial.[247] However, because no data appear to link preoperative treatment of Mg deficiency with benefit, screening with tests appears superfluous. Let us expand on this concept.
Hypomagnesemia is a prevalent laboratory finding in hospitalized patients (11% to 16%; Table 25-18 ). The total serum level of Mg represents the protein-bound (physiologically inactive) Mg, as well as the ionized (physiologically active) Mg. (Total serum Mg constitutes less than 1% of total body Mg, there being no constant ratio between the two values.)
|
|
|
|
Percentage with Abnormalities of | Percentage with Surgically Significant Abnormalities of | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | Series | Patients Examined (n) | PT | PTT | PLT CNT | BT | PT | PTT | PLT CNT | BT |
| ?<18 | Asaf et al[123] | 416 | 32.4 (121/373) | 17.6 (61/346) |
|
|
0 (0/416) |
|
|
|
| ?0–90 | Robbins and Rose[157] | 1,025 |
|
14 (143/1,025) |
|
|
0 (0/1,025) |
|
|
|
| Unspecified | Baranetsky and Weinstein[237] | 2,600 | 0.2 (5/2,600) |
|
|
0 (0/2,600) |
|
|
||
| Unspecified | Barber et al[238] | 1,941 (including 141 with risk factors) |
|
|
|
6 (110/1,941) * |
|
|
?2.2 (43/1,941) * | |
|
|
|
1,800 |
|
|
|
1.5 (27/1,800) * |
|
|
0.19 (2/1,800) * | |
| ?0–90 | Blery et al[128] | ∼2,900 | 0.1 (4/2,931) | 0.1 (4/2,914) | 0.3 (10/3,546) | 0.4 (17/3,845) | 0 (0/2,931) | 0 (0/2,914) | ?0.03 (1/3,576) | 0 (0/3,845) |
| 12–90 | Alsumait et al[125] | 1000 | 1.4 (14/1000) | 1.2 (12/1000) | 1.1 (11/1000) |
|
0 (0/1000) | 0 (0/1000) | 0 (0/1000) |
|
| ?0.25–65 | Narr et al[110] | 3,782 |
|
|
1.2 (40/3,782) |
|
|
|
0 (0/3,782) |
|
| 0–93 | Lorenzi and Cohen (personal communication) | 578 | 3.5 (20/578) |
|
|
0 (0/578) |
|
|
||
| >18 | Kaplan et al[111] | 154 |
|
|
|
|
0 (0/154) |
|
|
|
| ?>18 | Macpherson et al[239] | 1,109 | 1.8 (12/668) | 2.8 (29/1,008) | 10.1 (108/1,022) |
|
0 (0/668) | 0 (0/1,008) | 0 (0/1,022) |
|
| >18 | Rohrer et al[121] | 282 |
|
|
|
|
|
|
|
|
|
|
With risk factors |
|
0.6 (1/159) | 6.3 (10/159) | 8.2 (20/117) | 7.4 (14/170) | 0.6 (1/154) | ?1.4 (?3/159) | ?2.6 (?3/117) | ? |
|
|
Asymptomatic |
|
0.8 (1/123) | 2.4 (3,123) | 8.0 (13/103) | 3.8 (4/105) | 0 (0/123) | 0 (0/123) | 0 (0/103) | 0 (0/105) |
|
|
|
|
|
|
|
|
(0/154) |
|
|
|
| 32–90 | Johnson and Mortimer[124] | 100 |
|
|
6 (6/100) |
|
|
|
0 (0/100) |
|
| ≥71 | Levinstein et al[156] | 121 |
|
|
4.0 |
|
|
|
|
|
| Unspecified | Turnbull and Buck[113] | 1,005 | 0 (0/213) | 1.5 (3/210) | 0 (0/1,005) |
|
0 (0/123) | 0 (0/210) | 0 (0/1,005) |
|
| Unspecified | Bushnick et al[240] | 640 | 0.8 (5/591) | 0.3 (2/640) |
|
|
|
|
|
|
| Unspecified | Eisenberg et al[241] | 750 |
|
|
|
|
0 (0/750) |
|
|
|
| Unspecified | Muskett and McGreevy[115] | 200 | 3.9 (5/128) | 3.9 (5/126) |
|
|
0 (0/128) | 0 (0/126) |
|
|
| Unspecified | Suchman and Mushlin[242] | 1,004 |
|
|
|
|
|
|
|
|
|
|
With known risk factors |
|
|
10.4 (104/1,004) |
|
|
|
4.6 (46/1,004) |
|
|
|
|
Without known risk factors | 11,334 | 13.3 (243/1,927) |
|
|
|
0.71 † (13/1,827) |
|
|
|
| BT, bleeding time; PLT CNT, platelet count; PT, prothrombin time; PTT, partial thromboplastin time. | ||||||||||
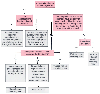 Figure 25-5
Procedure for determining whether coagulation tests are
needed. PT, prothrombin time; PTT, partial thromboplastin time. (Redrawn
and modified from Roizen MF, Hurd MJ: Preoperative patient evaluation. In
Rogers MC (ed): Current Practice in Anesthesiology, 2nd ed. St. Louis, Mosby-Year
Book, 1990, p 14.)
Figure 25-5
Procedure for determining whether coagulation tests are
needed. PT, prothrombin time; PTT, partial thromboplastin time. (Redrawn
and modified from Roizen MF, Hurd MJ: Preoperative patient evaluation. In
Rogers MC (ed): Current Practice in Anesthesiology, 2nd ed. St. Louis, Mosby-Year
Book, 1990, p 14.)
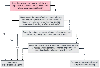 Figure 25-6
Suggested procedure for screening patients at high risk
for malignant hyperthermia.
Figure 25-6
Suggested procedure for screening patients at high risk
for malignant hyperthermia.
|
|
Wong et al[247] | Whang and Ryder[254] | Lum[255] | Reinhart and Desbiens[250] | Chernow et al[256] |
|---|---|---|---|---|---|
| Setting | LA County/USC Medical Center | Urban primary care hospital | VAMC | Community referral hospital | Mass. Gen. Hosp. |
| Patient selection criteria | Random; hospitalized patients | Random; hospitalized patients | Acute medical/surgical patients | Consecutive medical ICU patients | Postoperative ICU patients * |
| No. of subjects | 621 | 1,033 † | 353 | 102 | 193 |
| Hypomagnesemia |
|
|
|
|
|
| Definition | <1.2 mEq/L | <0.74 mmol/L | <0.72 mmol/L | <1.4 mEq/L | <1.5 mEq/L |
| Prevalence ‡ | 11.0% | 47.1% | 26.1% | 20% | 61% |
| Technique for measuring serum Mg | ACA, or AAS on a Perkin Elmer 560 B | ACA | ACA III | AAS | AAS (Varian 1275) |
| AAS, atomic absorption spectrophotometry; ACA, Du Pont Automatic Clinical Analyzer; ICU, intensive care unit; LA, Los Angeles; Mass. Gen. Hosp., Massachusetts General Hospital, Boston; USC, University of Southern California, Los Angeles; VAMC, Veterans Affairs Merical Center. | |||||
| Courtesy of Dr. Allan Jo and colleagues. | |||||
Fanning and colleagues[248] showed that intravenous administration of Mg for 4 days after coronary artery bypass grafting decreased the incidence and severity of atrial fibrillation. Specifically, 14 patients in the control group had 42 episodes of fibrillation, 2 of whom required cardioversion. In contrast, 7 patients in the Mg-treated group had 12 episodes of atrial fibrillation, and none required cardioversion. In a similar study, intraoperative administration of Mg after cardiopulmonary bypass decreased the frequency of postoperative ventricular arrhythmias (8 of 50 [16%] in the Mg-treated group versus 17 of 50 [34%] in the placebo-treated group, P < 0.04).[249]
Some investigators believe that serum Mg should be measured routinely in hospitalized patients because of the high prevalence of hypomagnesemia coupled with the difficulty of diagnosing hypomagnesemia on clinical grounds alone.[247] [250] One investigator argues that common sense dictates that nonessential surgery be deferred until Mg depletion has been corrected, again with no data to support such an assertion.[251] I could not find any data indicating better perioperative outcome in patients not undergoing cardiovascular surgery because of routine detection and correction of hypomagnesemia. The benefit of decreased frequency of cardiac dysrhythmias after cardiac surgery[248] [249] occurred with or without preoperative hypomagnesemia. If one is going to use magnesium empirically, one probably should have a measure of renal function and should monitor magnesium levels (or reflexes in the awake patient), but no data indicate that such levels should be routinely determined before surgery.
The National Veterans Affairs Surgical Risk Study found that albumin levels were an important predictor of outcome after surgery.[61] [222] Furthermore, changes in this level after enteral nutrition have been important predictors of perioperative outcome in malnourished or otherwise very sick patients (see Chapter 27 ). It may therefore be time to add this laboratory test for patients undergoing surgical class C procedures, and for patients who have a physiologic age (RealAge) of over 85 who are undergoing surgical class B procedures.
Even sophisticated laboratory tests have not been better in controlled trials than the history and physical examination in estimating the risk from a diagnosis. This lack of benefit from laboratory testing has applied to diagnoses as relatively amenable to laboratory diagnosis as the differential diagnosis of systolic murmurs, [252] assessment of nutritional status,[253] and diagnosis of cardiac and gastrointestinal disease.[89]
|
|
|
|
|
|
|
|
|
|
|
|
|