Classic Action of Depolarizing Muscle Relaxants
Depolarizing relaxants, at least initially, simulate the effect
of acetylcholine and therefore can be considered agonists despite the fact that they
block neurotransmission after initial stimulation. Structurally, succinylcholine
is two molecules of acetylcholine bound together. It is therefore not surprising
that it can mimic the effects of acetylcholine. Succinylcholine or decamethonium
can bind to the receptor, open the channel, pass current, and depolarize the end
plate. These agonists, similar to acetylcholine, attach only briefly; each opening
of a channel is of very short duration, 1 millisecond or less. The response to acetylcholine,
however, is over in milliseconds because of its rapid degradation by acetylcholinesterase,
and the end plate resets to its resting state long before another nerve impulse arrives.
In contrast, the depolarizing relaxants characteristically have a biphasic action
on muscle—an initial contraction, followed by relaxation lasting minutes to
hours. The depolarizing relaxants, because they are not susceptible to hydrolysis
by acetyl-cholinesterase, are not eliminated from the junctional cleft until after
they are eliminated from the plasma. The time required to clear the drug from the
body is the principal determinant of how long the drug effect lasts. Whole-body
clearance of the relaxant is very slow compared with acetylcholine, even when the
plasma cholinesterase is normal. Because relaxant molecules are not cleared from
the cleft quickly, they react repeatedly with receptors, attaching to one almost
immediately after separating from another, thereby repeatedly depolarizing the end
plate and opening channels.
The quick shift from excitation of muscle contraction to blockade
of transmission by depolarizing relaxants occurs because the end plate is continuously
depolarized. This comes about because of the juxtaposition at the edge of the end
plate on the muscle membrane—a different kind of ion channel, the sodium channel,
that does not respond to chemicals but opens when exposed to a transmembrane voltage
change. The sodium channel is also a cylindrical transmembrane protein through which
sodium ions can flow. Two parts of its structure act as gates that allow or stop
the flow of sodium ions.[50]
Both gates must be
open if sodium is to flow through the channel; the closing of either cuts off the
flow. Because these two gates act sequentially, a sodium channel has three functional
conformation states and can move progressively from one state to another ( Fig.
22-7
).
When the sodium channel is in its resting state, the lower gate
(i.e., the time-dependent or inactivation gate) is open, but the upper gate (i.e.,
the voltage-dependent gate) is closed, and sodium ions cannot pass. When the molecule
is subject to a sudden change in voltage by depolarization of the adjacent membrane,
the top gate opens, and because the bottom (time-dependent) gate is still open, sodium
flows through the channel. The voltage-dependent gate stays open as long as the
molecule is subject to a depolarizing influence from the membrane around it; it will
not close until the depolarization disappears. However, shortly after the voltage-dependent
gate opens, the bottom gate closes and again cuts off the flow of ions. It cannot
open again until the voltage-dependent gate closes. When the depolarization of the
end plate stops, the voltage-dependent gate closes, the time-dependent one opens,
and the sodium channel returns to its resting state. This whole process is short
lived when depolarization occurs with acetylcholine. The initial response of a depolarizing
muscle relaxant resembles that of acetylcholine, but because the relaxant is not
hydrolyzed rapidly, depolarization of the end plate is not brief.
Depolarization of the end plate by the relaxant initially causes
the voltage gate in adjacent sodium channels to open, causing a wave of depolarization
to sweep along
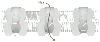 Figure 22-7
Sketch of sodium channel. The bars
represent parts of the molecule that act as gates. The upper bar is voltage dependent;
the lower bar is time dependent. The left side of the drawing represents the resting
state. Once activated by a voltage change, the molecule and its gates progress as
illustrated (left to right).
Figure 22-7
Sketch of sodium channel. The bars
represent parts of the molecule that act as gates. The upper bar is voltage dependent;
the lower bar is time dependent. The left side of the drawing represents the resting
state. Once activated by a voltage change, the molecule and its gates progress as
illustrated (left to right).
the muscle, producing muscle contraction. Shortly after the voltage-dependent gate
opens, the time-dependent inactivation gate closes. Because the relaxant is not
removed from the cleft, the end plate continues to be depolarized. Because the sodium
channels immediately adjacent to the end plate are influenced by the depolarization
of the end plate, their voltage-dependent gates stay open, and their inactivation
gates stay closed. Because sodium cannot flow through a channel that has a closed
inactivation gate, the perijunctional muscle membrane does not depolarize. When
the flow of ions though the sodium channels in the perijunctional zone stops because
the inactivation gates have closed, the channels down-stream (beyond the perijunctional
zone) are freed of depolarizing influence. In effect, the perijunctional zone becomes
a buffer that shields the rest of the muscle from events at the end plate. Consequently,
the muscle membrane is separated into three zones: the end plate, which is depolarized
by succinylcholine; the perijunctional muscle membrane, in which the sodium channels
are frozen in an inactivated state; and the rest of the muscle membrane, in which
the sodium channels are in the resting state. Because a burst of acetylcholine from
the nerve cannot overcome the inactivated sodium channels in the perijunctional zone,
neuromuscular transmission is blocked. This phenomenon is also called accommodation.
During accommodation, when the synapse is inexcitable through the nerve (transmitter),
direct electrical stimulation of muscle causes muscle contraction because the sodium
channels beyond the junctional area are in the resting excitable state.
The extraocular muscles contain tonic muscle, which is multiply
innervated and chemically excitable along most of its surface.[15]
Accommodation does not occur, and these muscles can undergo a sustained contracture
in the presence of succinylcholine. The tension so developed forces the eye against
the orbit and accounts for part of the increase in intraocular pressure produced
by depolarizing relaxants. There is also evidence that the extraocular muscles contain
a special type of receptor that does not become desensitized (discussed later) in
the continued presence of acetylcholine or other agonists.[51]
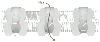 Figure 22-7
Sketch of sodium channel. The bars
represent parts of the molecule that act as gates. The upper bar is voltage dependent;
the lower bar is time dependent. The left side of the drawing represents the resting
state. Once activated by a voltage change, the molecule and its gates progress as
illustrated (left to right).
Figure 22-7
Sketch of sodium channel. The bars
represent parts of the molecule that act as gates. The upper bar is voltage dependent;
the lower bar is time dependent. The left side of the drawing represents the resting
state. Once activated by a voltage change, the molecule and its gates progress as
illustrated (left to right).