Basic Electrophysiology of Neurotransmission
Progress in electrophysiologic techniques has moved at an equal
pace with advances in molecular approaches to study the receptor. Patch-clamping
is a technique in which a glass micropipette is used to probe the membrane surface
until a single functional receptor is encompassed. The tip of the pipette is pressed
into the lipid of the membrane, and the electronic apparatus is arranged to keep
the membrane potential clamped (i.e., fixed) and to measure the current that flows
through the channel of the receptor. The solution in the pipette can contain acetylcholine,
tubocurarine, another drug, or a mixture of drugs. By application of these drugs
to the receptor through the micropipette, electrical changes can be monitored.
Figure 22-6
illustrates the results of the classic depolarizing action of acetylcholine on end-plate
receptors. Normally, the pore of the channel is closed by the
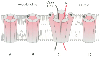 Figure 22-6
The actions of acetylcholine or curare on end-plate receptors.
A, The ion channel is inactive and does not open
in the absence of acetylcholine. B, Even the binding
of one acetylcholine molecule (filled circle) to
one of two binding sites does not open the channel. C,
When acetylcholine binds to recognition sites of both α-subunits simultaneously
(filled circle), it triggers a conformation change
that opens the channel and allows ions to flow across the membrane. D,
The action of antagonists such as curare (filled square).
Acetylcholine is in competition with tubocurarine for the receptor's recognition
site but may also react with acetylcholinesterase. Inhibiting the enzyme increases
the lifetime of acetylcholine and the probability that it will react with a receptor.
When one of the two binding (recognition) sites is occupied by curare, the receptor
will not open, even if the other binding site is occupied by acetylcholine.
Figure 22-6
The actions of acetylcholine or curare on end-plate receptors.
A, The ion channel is inactive and does not open
in the absence of acetylcholine. B, Even the binding
of one acetylcholine molecule (filled circle) to
one of two binding sites does not open the channel. C,
When acetylcholine binds to recognition sites of both α-subunits simultaneously
(filled circle), it triggers a conformation change
that opens the channel and allows ions to flow across the membrane. D,
The action of antagonists such as curare (filled square).
Acetylcholine is in competition with tubocurarine for the receptor's recognition
site but may also react with acetylcholinesterase. Inhibiting the enzyme increases
the lifetime of acetylcholine and the probability that it will react with a receptor.
When one of the two binding (recognition) sites is occupied by curare, the receptor
will not open, even if the other binding site is occupied by acetylcholine.
approximation of the cylinders (i.e., subunits). When an agonist occupies both α-subunit
sites, the protein molecule undergoes a conformational change that forms a channel
in the center through which ions can flow along a concentration gradient (see Fig.
22-6
). When the channel is open, there is a flow of sodium and calcium
from the outside of the cell to the inside and of potassium from the inside to the
outside. The channel in the tube is large enough to accommodate many cations and
electrically neutral molecules, but it excludes anions (e.g., chloride). The current
carried by the ions depolarizes the adjacent membrane. The net current is depolarizing
and creates the end-plate potential that stimulates the muscle to contract. In this
instance, downward-going (i.e., depolarizing) rectangular pulses (see Fig.
22-4
) can be recorded by the electrophysiologic technique described previously.
The pulse stops when the channel closes and one or both agonist
molecules detach from the receptor. The current that passes through each open channel
is minuscule, only a few picoamperes (about 104
ions/msec). However,
each burst of acetylcholine from the nerve normally opens about 500,000 channels
simultaneously, and the total current is more than adequate to produce depolarization
of the end plate and contraction of muscle. The opening of a channel causes the
conversion of chemical signals from a nerve to current flows to end-plate potentials,
leading to muscle contraction. We are used to thinking of the end-plate potential
as a graded event, which may be reduced in magnitude or extended in time by drugs,
but in reality, the end-plate potential is the summation of many all-or-nothing events
occurring simultaneously at myriad ion channels. It is these tiny events that are
affected by drugs.
Receptors that do not have two molecules of agonists bound remain
closed. Both α-subunits must be occupied simultaneously by agonist; if only
one of them is occupied, the channel remains closed (see Fig.
22-6
). This is the basis for the prevention of depolarization by antagonists.
Drugs such as tubocurarine act by binding to either or both α-subunits, preventing
acetylcholine from binding and opening the channel. This interaction between agonists
and antagonists is competitive, and the outcome—transmission or block—depends
on the relative concentrations and binding characteristics of the drugs involved
(see "Drug Effects on Postjunctional Receptors").
Individual channels are also capable of a wide variety of conformations.
[47]
[48]
They
may
open or stay closed, affecting total current flow across the membrane, but they can
do more. They may open for a longer or shorter time than normal, open or close more
gradually than usual, open briefly and repeatedly (i.e., chatter), or pass fewer
or more ions per opening than they usually do. Their function also is influenced
by drugs, changes in the fluidity of the membrane, temperature, the electrolyte balance
in the milieu, and other physical and chemical factors.[49]
Receptor channels are dynamic structures that are capable of a wide variety of interactions
with drugs and of entering a wide variety of current-passing states. All these influences
on channel activity ultimately are reflected in the strength or weakness of neuromuscular
transmission and the contraction of a muscle.
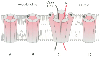 Figure 22-6
The actions of acetylcholine or curare on end-plate receptors.
A, The ion channel is inactive and does not open
in the absence of acetylcholine. B, Even the binding
of one acetylcholine molecule (filled circle) to
one of two binding sites does not open the channel. C,
When acetylcholine binds to recognition sites of both α-subunits simultaneously
(filled circle), it triggers a conformation change
that opens the channel and allows ions to flow across the membrane. D,
The action of antagonists such as curare (filled square).
Acetylcholine is in competition with tubocurarine for the receptor's recognition
site but may also react with acetylcholinesterase. Inhibiting the enzyme increases
the lifetime of acetylcholine and the probability that it will react with a receptor.
When one of the two binding (recognition) sites is occupied by curare, the receptor
will not open, even if the other binding site is occupied by acetylcholine.
Figure 22-6
The actions of acetylcholine or curare on end-plate receptors.
A, The ion channel is inactive and does not open
in the absence of acetylcholine. B, Even the binding
of one acetylcholine molecule (filled circle) to
one of two binding sites does not open the channel. C,
When acetylcholine binds to recognition sites of both α-subunits simultaneously
(filled circle), it triggers a conformation change
that opens the channel and allows ions to flow across the membrane. D,
The action of antagonists such as curare (filled square).
Acetylcholine is in competition with tubocurarine for the receptor's recognition
site but may also react with acetylcholinesterase. Inhibiting the enzyme increases
the lifetime of acetylcholine and the probability that it will react with a receptor.
When one of the two binding (recognition) sites is occupied by curare, the receptor
will not open, even if the other binding site is occupied by acetylcholine.