Morphology
The neuromuscular junction is specialized on the nerve side and
on the muscle side to transmit and receive chemical messages.[8]
[9]
[10]
[11]
[12]
Each motor neuron runs without interruption
from the ventral horn of the spinal cord to the neuromuscular junction as a large,
myelinated axon. As it approaches the muscle, it branches repeatedly to contact
many muscle cells, to gather them into a functional group known as a motor
unit. The architecture of the nerve terminal is quite different from
that of the rest of the axon. As the terminal reaches the muscle fiber, it loses
its myelin to form a spray of terminal branches against the muscle surface and is
covered by Schwann cells.[10]
This arrangement
conforms to the architecture on the synaptic area of muscle membrane ( Fig.
22-1
). The nerve is separated from the surface of the muscle by a gap
of approximately 20 nm, called the junctional cleft.
The nerve and muscle are held in tight alignment by protein filaments called basal
lamina, which span the cleft between nerve and end plate. The muscle surface is
heavily corrugated, with deep invaginations of the junctional cleft—the primary
and secondary clefts—between the folds in the muscle membrane; the end plate's
total surface area is very large. The depths of the folds also vary between muscle
types and species. The human neuromuscular junctions, relative to muscle size, are
smaller than those of the mouse, although the junctions are located on muscle fibers
that are much larger. Human junctions have longer junctional foldings and deeper
gutters.[11]
The functional significance of these
folds is unclear. The shoulders of the folds are densely populated with acetylcholine
receptors, about 5 million of them in each junction. These receptors are sparse
in the depths between the folds. Instead, these deep areas contain sodium channels.
The trophic function of the nerve is vital for the development
and maintenance of adequate neuromuscular function. Before birth, each muscle cell
commonly has contacts with several nerves and has several neuromuscular junctions.
[14]
At birth, all but one of the nerves retract,
and a single end plate remains. Once formed, the nerve-muscle contact, especially
the end plate, is durable. Even if the original nerve dies, the one replacing it
innervates exactly the same region of the muscle. The nerve endings on fast muscles
are larger and more complicated than those on slow muscles. The reason for this
is unclear. These differences in the nerve endings on the muscle surfaces may play
a role in the differences in the response to muscle relaxants of fast and slow muscles.
Because all the muscle cells in a unit are excited by a single
neuron, stimulation of the nerve electrically or by an action potential originating
from the ventral horn or by any agonist, including depolarizing relaxants (e.g.,
succinylcholine), causes all muscle cells in the motor unit to contract synchronously.
The synchronous contraction of the cells in a motor unit is fasciculation and often
is vigorous enough to be observed through the skin. Although most adult human muscles
have only one neuromuscular junction per cell, an important exception is some of
the cells in the extraocular muscles. The extraocular muscles are "tonic" muscles,
and unlike other mammalian striated muscles, they are multiply innervated, with several
neuromuscular junctions strung along the surface of each muscle cell.[15]
These muscles contract and relax slowly, rather than quickly as other striated muscles
do; they can maintain a steady contraction, or contracture, whose strength is proportional
to the stimulus received.
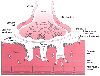 Figure 22-1
Adult neuromuscular junction with the three cells that
constitute the synapse: the motor neuron (i.e., nerve terminal), muscle fiber, and
Schwann cell. The motor neuron from the ventral horn of the spinal cord innervates
the muscle. Each fiber receives only one synapse. The motor nerve loses its myelin
to terminate on the muscle fiber. The nerve terminal, covered by a Schwann cell,
has vesicles clustered about the membrane thickenings, which are the active zones,
toward its synaptic side and mitochondria and microtubules toward its other side.
A synaptic gutter, made up of a primary and many secondary clefts, separates the
nerve from the muscle. The muscle surface is corrugated, and dense areas on the
shoulders of each fold contain acetylcholine receptors. The sodium channels are
present at the clefts and throughout muscle membrane.
Figure 22-1
Adult neuromuscular junction with the three cells that
constitute the synapse: the motor neuron (i.e., nerve terminal), muscle fiber, and
Schwann cell. The motor neuron from the ventral horn of the spinal cord innervates
the muscle. Each fiber receives only one synapse. The motor nerve loses its myelin
to terminate on the muscle fiber. The nerve terminal, covered by a Schwann cell,
has vesicles clustered about the membrane thickenings, which are the active zones,
toward its synaptic side and mitochondria and microtubules toward its other side.
A synaptic gutter, made up of a primary and many secondary clefts, separates the
nerve from the muscle. The muscle surface is corrugated, and dense areas on the
shoulders of each fold contain acetylcholine receptors. The sodium channels are
present at the clefts and throughout muscle membrane.
Physiologically, this specialization apparently holds the eye steadily in position.
These muscles are important to an anesthetist because depolarizing relaxants affect
them differently than they do most skeletal muscles. Instead of causing a brief
contraction followed by paralysis, the drugs cause a long-lasting contracture response,
which pulls the eye against the orbit and contributes to an increase in the pressure
of the intraocular fluid[16]
(see Chapter
65
). The clinical significance of this has been questioned. Although
many textbooks invoke the reported extrusion of intraocular content with succinylcholine,
the basis for this seems to be anecdotal.[17]
The perijunctional zone is the area of muscle immediately beyond
the junctional area, and it is critical to the function of the neuromuscular junction.
The perijunctional zone contains a mixture of the receptors, which include a smaller
density of acetylcholine receptors and high-density sodium channels (see Fig.
22-1
). The admixture enhances the capacity of the perijunctional zone
to respond to the depolarization (i.e., end-plate potential) produced by acetylcholine
receptors and to transduce it into the wave of depolarization that travels along
the muscle to initiate muscle contraction. The density of sodium channels in the
perijunctional area is richer than in more distal parts of the muscle membrane.[18]
The perijunctional zone is close enough to the nerve ending to be influenced by
transmitter released from it. Moreover, special variants (i.e., isoforms) of receptors
(see "Biology of Prejunctional and Postjunctional Nicotinic Receptors") and sodium
channels can appear in this area at different stages of life and in response to abnormal
decreases in nerve activity. Congenital abnormalities in the acetylcholine receptor
[3]
or the sodium channels (i.e., mutations)[19]
are also known. These variabilities seem to contribute to the differences in response
to relaxants that are seen in patients with different pathologic conditions and ages.
[1]
[20]
Such qualitative
differences may also play a role in altered muscle function (see "Myopathy of Critical
Illness and Acetylcholine Receptors").
 |
 Figure 22-1
Adult neuromuscular junction with the three cells that
constitute the synapse: the motor neuron (i.e., nerve terminal), muscle fiber, and
Schwann cell. The motor neuron from the ventral horn of the spinal cord innervates
the muscle. Each fiber receives only one synapse. The motor nerve loses its myelin
to terminate on the muscle fiber. The nerve terminal, covered by a Schwann cell,
has vesicles clustered about the membrane thickenings, which are the active zones,
toward its synaptic side and mitochondria and microtubules toward its other side.
A synaptic gutter, made up of a primary and many secondary clefts, separates the
nerve from the muscle. The muscle surface is corrugated, and dense areas on the
shoulders of each fold contain acetylcholine receptors. The sodium channels are
present at the clefts and throughout muscle membrane.
Figure 22-1
Adult neuromuscular junction with the three cells that
constitute the synapse: the motor neuron (i.e., nerve terminal), muscle fiber, and
Schwann cell. The motor neuron from the ventral horn of the spinal cord innervates
the muscle. Each fiber receives only one synapse. The motor nerve loses its myelin
to terminate on the muscle fiber. The nerve terminal, covered by a Schwann cell,
has vesicles clustered about the membrane thickenings, which are the active zones,
toward its synaptic side and mitochondria and microtubules toward its other side.
A synaptic gutter, made up of a primary and many secondary clefts, separates the
nerve from the muscle. The muscle surface is corrugated, and dense areas on the
shoulders of each fold contain acetylcholine receptors. The sodium channels are
present at the clefts and throughout muscle membrane.