BASIC PHARMACOLOGY
Chemistry
The Local Anesthetic Molecule
The typical local anesthetic molecule, exemplified by lidocaine
and procaine ( Fig. 14-1
),
contains a tertiary amine attached to a substituted aromatic ring by an intermediate
chain. The tertiary amine is a base (proton acceptor). The chain almost always
contains either an ester(
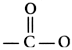
)
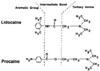 Figure 14-1
Structures of two local anesthetics, the aminoamide lidocaine
and the aminoester procaine. In both drugs a hydrophobic aromatic group is joined
to a more hydrophilic base, the tertiary amine, by an intermediate amide or ester
bond.
Figure 14-1
Structures of two local anesthetics, the aminoamide lidocaine
and the aminoester procaine. In both drugs a hydrophobic aromatic group is joined
to a more hydrophilic base, the tertiary amine, by an intermediate amide or ester
bond.
or amide (
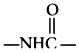
) linkage; local anesthetics may therefore be
classified as aminoester or aminoamide compounds. The aromatic ring system gives
a lipophilic (membrane-liking) character to its portion of the molecule, whereas
the tertiary amine end is relatively hydrophilic, particularly because it is partially
protonated and thus bears some positive charge in the physiologic pH range ( Fig.
14-2
). The structures of commonly administered local anesthetics are given
in Table 14-1
and their physicochemical
properties in Table 14-2
.
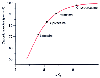 Figure 14-2
The fraction of local anesthetic in the protonated, cationic
form in aqueous solution at physiologic pH (7.4) as a function of the pKa
of the drug. The drug with the lowest pKa
,
lidocaine, has the smallest fraction of its molecules protonated and the largest
in the neutral form, and vice versa for the local anesthetic with the highest pKa
,
chloroprocaine. Individual drug molecules become protonated and deprotonated in
thousandths of a second in solution.
Figure 14-2
The fraction of local anesthetic in the protonated, cationic
form in aqueous solution at physiologic pH (7.4) as a function of the pKa
of the drug. The drug with the lowest pKa
,
lidocaine, has the smallest fraction of its molecules protonated and the largest
in the neutral form, and vice versa for the local anesthetic with the highest pKa
,
chloroprocaine. Individual drug molecules become protonated and deprotonated in
thousandths of a second in solution.
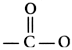
 Figure 14-1
Structures of two local anesthetics, the aminoamide lidocaine
and the aminoester procaine. In both drugs a hydrophobic aromatic group is joined
to a more hydrophilic base, the tertiary amine, by an intermediate amide or ester
bond.
Figure 14-1
Structures of two local anesthetics, the aminoamide lidocaine
and the aminoester procaine. In both drugs a hydrophobic aromatic group is joined
to a more hydrophilic base, the tertiary amine, by an intermediate amide or ester
bond.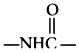
 Figure 14-2
The fraction of local anesthetic in the protonated, cationic
form in aqueous solution at physiologic pH (7.4) as a function of the pKa
of the drug. The drug with the lowest pKa
,
lidocaine, has the smallest fraction of its molecules protonated and the largest
in the neutral form, and vice versa for the local anesthetic with the highest pKa
,
chloroprocaine. Individual drug molecules become protonated and deprotonated in
thousandths of a second in solution.
Figure 14-2
The fraction of local anesthetic in the protonated, cationic
form in aqueous solution at physiologic pH (7.4) as a function of the pKa
of the drug. The drug with the lowest pKa
,
lidocaine, has the smallest fraction of its molecules protonated and the largest
in the neutral form, and vice versa for the local anesthetic with the highest pKa
,
chloroprocaine. Individual drug molecules become protonated and deprotonated in
thousandths of a second in solution.