|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The development of the neuromuscular junction is not complete at birth.[17] [656] In humans, maturation of neuromuscular transmission probably occurs after the first 2 months of age.[657] Nonetheless, neuromuscular blockers can be used safely in term and preterm infants.
Routine administration of succinylcholine to healthy children should be discontinued. In apparently healthy children, intractable cardiac arrest with hyperkalemia, rhabdomyolysis, and acidosis may develop after succinylcholine administration, particularly in patients with unsuspected muscular dystrophy of the Duchenne type.[658] [659] In response to this potential adverse effect, the U.S. Food and Drug Administration and Glaxo-Wellcome have modified the package insert for succinylcholine by adding a warning against the use of succinylcholine in children except for emergency control of the airway (see the section on complications of succinylcholine; also see Chapter 60 ).
It is not apparent from older studies whether newborns are more sensitive than adults to nondepolarizing neuromuscular blockers.[660] [661] [662] [663] More recent studies by Fisher and colleagues[241] [664] [665] on the pharmacokinetics and pharmacodynamics of neuromuscular blockers in infants, children, and adults, however, have made it possible to better understand the clinical pharmacology of these drugs in pediatric patients (see Chapter 60 ). Neonates and infants are more sensitive than adults to the neuromuscular blocking effects of dTc.[664] A lower plasma concentration of this neuromuscular blocker is required to achieve a desired level of neuromuscular blockade in these young patients. However, the dosage should not be decreased because infants have a larger volume of distribution. The increased volume of distribution and slower clearance ( Fig. 13-33 ) contribute to a longer elimination half-life,[664] [666] which
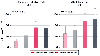 Figure 13-33
Correlation between age, glomerular filtration, and clearance
of d-tubocurarine (dTc). (Redrawn from Fisher
DM, O'Keeffe C, Stanski DR, et al: Pharmacokinetics and pharmacodynamics of d-tubocurarine
in infants, children, and adults. Anesthesiology 57:203–208, 1982.)
Figure 13-33
Correlation between age, glomerular filtration, and clearance
of d-tubocurarine (dTc). (Redrawn from Fisher
DM, O'Keeffe C, Stanski DR, et al: Pharmacokinetics and pharmacodynamics of d-tubocurarine
in infants, children, and adults. Anesthesiology 57:203–208, 1982.)
Atracurium, vecuronium, cisatracurium, rocuronium, and mivacurium are commonly administered to children. The popularity of these drugs in children most likely stems from the following points: minimal residual paralysis is seen in the postoperative period,[667] [668] and a faster onset of action occurs in children than in adults.
Atracurium and vecuronium, in comparison, show very different kinetic and dynamic patterns in infants. As with the long-acting neuromuscular blockers, the sensitivity of infants to vecuronium is greater than it is in children (ED95 of 0.047 versus 0.081 mg/kg, respectively).[669] The increased duration of action in infants is most likely secondary to the increased volume of distribution of vecuronium because its clearance is unchanged.[665] [666] Vecuronium therefore acts as a long-acting neuromuscular blocker in neonates.[665] [666] [670]
In contrast, the duration of action of atracurium is not significantly different in pediatric patients than it is in adults.[671] [672] [673] As with vecuronium and dTc, the volume of distribution is increased in infants.[241] However, clearance of atracurium is also more rapid.[241] Therefore, the same dose (0.5 to 0.6 mg/kg) can be used in infants, children, and adults for tracheal intubation without any major difference in its duration of action in the three groups. In children, a dose of 0.1 mg/kg cisatracurium has an onset of just over 2 minutes and a clinical duration of approximately 30 minutes during balanced or halothane anesthesia.[674] The calculated ED95 doses of cisatracurium in infants and children are 43 and 47 mg/kg, respectively.[675] The mean infusion rate necessary to maintain 90% to 99% neuromuscular blockade is also similar in infants and children. [676]
Rocuronium in adults is an intermediate-acting neuromuscular blocker with a fast onset of action, and the same is also true in infants and children.[676] [677] Its potency is greater in infants than children, but its onset is faster in the latter age group.[677] In children, rocuronium, 0.6 mg/kg, produces better conditions for rapid tracheal intubation than vecuronium, 0.1 mg/kg, or atracurium, 0.5 mg/kg, does.[676] If given by intramuscular
The ED95 of mivacurium is greater in children than adults (0.10 mg/kg during narcotic anesthesia and 0.09 mg/kg during halothane anesthesia). [680] Children therefore require larger doses of mivacurium than adults do to achieve a given depth of neuromuscular blockade.[680] [681] Onset time, as with atracurium[670] and vecuronium,[682] is faster in children than adults, so maximal block is achieved in less than 2 minutes. A dose of 0.25 to 0.30 mg/kg facilitates tracheal intubation in about 90 seconds in children. A dose of twice the ED95 (0.2 mg/kg) results in only a 20% increase in the duration of blockade as compared with the ED95 .[681] In contrast to adults, large doses have less of a propensity to cause histamine release. Facial flushing and transient hypotension are observed in about 10% to 15% of the pediatric population who receive 0.25 mg/kg mivacurium as a rapid 5- to 10-second injection.[680] Mivacurium's clinical duration of action is shorter in children than adults (12 versus 15 to 20 minutes). Because of its short duration of action, mivacurium is best used as an infusion in children for maintenance of relaxation. Infusion rates required by children (10 to 20 µg/kg/min) are about twice those required by adults, probably because of significantly higher butyrylcholinesterase activity.[683] [684]
Antagonism of residual neuromuscular blockade in the case of the various nondepolarizers is similar in children and adults. Fisher and associates [629] [685] described some minor variations in the neostigmine and edrophonium dosage for pediatric patients. For example, the ED50 of neostigmine for antagonism of a dTc-induced 90% block of the adductor pollicis twitch was 22.9 µg/kg in adults versus 15.5 µg/kg in infants.[629] In the case of edrophonium, the ED50 for antagonism of a dTc-induced 90% block was 128 µg/kg in adults. In children, the ED50 was 233 µg/kg, and in infants the ED50 was 145 µg/kg.[685] The rate of recovery of intermediate- or short-acting neuromuscular blockers is faster than that of long-acting drugs in children.[583] [686]
Neostigmine doses of up to 50 to 60 µg/kg or edrophonium doses of 500 to 1000 µg/kg should be used for antagonism of residual neuromuscular blockade in children. In all cases, tests of clinical recovery, such as head lift, leg lift, and cry, should be performed and documented for pediatric patients and adults.
|
|
|
|
|
|
|
|
|
|
|
|
|