|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The direct cardiac actions of opioids, and in particular the effects on myocardinal contractile mechanisms, are significantly less than those of many other intravenous and inhalation anesthetics. However, opioid receptors have been demonstrated to exist in cardiac myocytes of several species.
Some investigators suggest that opioids produce direct positive inotropic effects on the heart.[180] However, others report negative inotropic actions with some agents (meperidine) and no direct effects with others (morphine).[181] Some investigators suggest that local anesthetic-like effects, and not receptor-related opioid actions, mediate some of the negative inotropic effects of opioids, especially with high drug concentrations.
Morphine decreases Ca2+ transients but not cardiac contraction, and enhances myofilament Ca2+ sensitivity, through an action on the δ1 opioid receptor expressed in the heart.[182] On the other hand, it was demonstrated that morphine decreases the isometric force of contraction in atrial muscle samples from non-failing and failing human hearts through a naloxone-insensitive mechanism.[183] Most evidence, however, indicates that fentanyl produces little or no change in myocardial contractility,[184] but it has also been reported that fentanyl has positive inotropic effects. Possible mechanisms of the dose-dependent positive inotropic effects of fentanyl, as well as of sufentanil, include catecholamine release or direct myocardial adrenergic activation. Usually, most hemodynamic variables remain unchanged after large doses of fentanyl. Little or no depression of cardiac index or pump function has been reported after sufentanil in humans.[184] Studies in dogs have demonstrated little change in hemodynamics with moderate doses (160 µg/kg) of alfentanil and transient cardiac stimulation (increases in left ventricular contractility, aortic blood flow velocity, and acceleration) with very large doses (5 mg/kg). In dogs, remifentanil produces hemodynamic effects that include decreases in contractility and cardiac output, as well as decreases in heart rate and blood pressure.[185]
Opioid-induced bradycardia is primarily mediated by the CNS. However, there have been reports of direct effects of opioids on cardiac pacemaker cells. Asystole may follow opioid-induced bradycardia. Premedication with or concomitant administration of, β-adrenergic or Ca2+ entry blockers can exacerbate bradycardias and may result in asystole after opioids. Periods of asystole, 10 to 12 seconds
Opioids may depress cardiac conduction. Fentanyl slows atrioventricular node conduction, and prolongs the RR interval, the atrioventricular node refractory period and Purkinje fiber action potential duration.[186] [187] These effects have been suggested to be mediated by direct membrane actions, as opposed to opioid receptor interactions.[188] Opioids can also prolong the QT interval.[189] However, both sufentanil and alfentanil have been demonstrated to be devoid of electrophysiologic effects on normal or accessory pathways in patients with Wolff-Parkinson-White syndrome. [190] [191]
Clinically, cardiac conduction disturbances due to opioids are very rare, but they may be more likely to occur in the presence of Ca2+ entry or β-adrenergic blockers.[192] Indeed, the overall effect of opioid anesthesia is anti-arrhythmic.[193] Antifibrillatory effects have been suggested to be produced by fentanyl.[194] Some of the electrophysiologic actions of opioids resemble those of class III anti-arrhythmic drugs. Opioid antagonists appear more antiarrhythmogenic than agonists in rats. [195]
Determining the effects and consequences of opioid action on myocardial ischemia is complex.[196] [197] [198] Results can depend on such factors as the species studied and the experimental design. Recently, it has been suggested that opioids can mimic ischemic preconditioning. It was demonstrated that opioid receptor stimulation results in a reduction in infarct size similar to that produced by ischemic preconditioning ( Fig. 11-9 ). [199] It was recently reported that stimulation of the δ1 -opioid receptor generates oxygen radicals via mitochondrial ATP-sensitive K+ channels, resulting in attenuation of oxidant stress and cell death in cardiomyocytes.[200] Involvement of the adenosine A1 receptor and protein kinase C in the cardioprotective effect of opioids was also suggested.[201] [202] Whether the experimental results showing protective effects of opioids against myocardial ischemia will translate into reductions in morbidity and mortality in patients with coronary artery disease has yet to be established by clinical trials.[203]
High doses of opioids can maintain myocardial perfusion and the O2 supply/demand ratio as well as or better than inhalation-based techniques. [204] [205] Among the opioids, alfentanil is associated with more myocardial ischemia (as indicated by reversal of the myocardial lactate extraction to production ratio and the worsening ventricular diastolic compliance) than either fentanyl or sufentanil in patients having coronary artery surgery.[206] Data from a prospective randomized comparison of sufentanil versus enflurane, isoflurane, and halothane for coronary artery surgery revealed no difference in new intraoperative ischemia, the incidence of postoperative myocardial infarction, or death.[207]
Opioids appear to have no significant effect on coronary vasomotion or myocardial metabolism, do not produce steal phenomena, and do not diminish the ability of
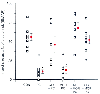 Figure 11-9
Infarct sizes in rat hearts subjected to control conditions
(CON), ischemic preconditioning (PC), glibenclamide (0.3 mg/kg IV) given 30 minutes
before ischemic PC (GLY + PC), morphine-induced PC (3 × 100 µg/kg,
5 minutes of IV infusion) (MOR PC), naloxone (3 mg/kg IV) given 10 minutes before
morphine-induced PC (NL + MOR PC), and glibenclamide (0.3 mg/kg IV) given 30 minutes
before morphine-induced PC (GLY + MOR PC). (From Schultz JE, Hsu AK, Gross
GJ: Morphine mimics the cardioprotective effect of ischemic preconditioning via
a glibenclamide-sensitive mechanism in the rat heart. Circ Res 78:1100–1104,
1996.)
Figure 11-9
Infarct sizes in rat hearts subjected to control conditions
(CON), ischemic preconditioning (PC), glibenclamide (0.3 mg/kg IV) given 30 minutes
before ischemic PC (GLY + PC), morphine-induced PC (3 × 100 µg/kg,
5 minutes of IV infusion) (MOR PC), naloxone (3 mg/kg IV) given 10 minutes before
morphine-induced PC (NL + MOR PC), and glibenclamide (0.3 mg/kg IV) given 30 minutes
before morphine-induced PC (GLY + MOR PC). (From Schultz JE, Hsu AK, Gross
GJ: Morphine mimics the cardioprotective effect of ischemic preconditioning via
a glibenclamide-sensitive mechanism in the rat heart. Circ Res 78:1100–1104,
1996.)
Potent opioids applied in anesthesia usually produce minimal to no decreases in preload and afterload, and little depression of great vessel and atrial baroreceptors,[210] in contrast to potent inhalation agents such as halothane.[211] Infants, after receiving 10 µg/kg of fentanyl, do demonstrate significant depression of baroreflex responses.[212]
Some authors have suggested that aortic root dissection causes hypertension mediated by serotonin via stimulation of a specific cardiogenic reflex. Although higher doses of fentanyl or sufentanil can decrease the occurrence of intraoperative hypertension, no dose of any opioid has been shown to prevent all hypertensive responses reliably, especially during cardiac surgery in humans.[59]
|
|
|
|
|
|
|
|
|
|
|
|
|