Opioid Receptors
In 1973, three independent teams of investigators described the
presence of opioid binding sites in the nervous system from radioligand binding assays.
Based on pharmacological experiments, three types of opioid receptors were postulated.
They were named μ for morphine type, κ for the ketocyclazocine type, and
σ for the SKF10047 (N-allylnormetazocine) type. In addition, a high-affinity
receptor for enkephalins was found in the mouse vas deferens, and named as a δ-receptor.
Furthermore, an epsilon-receptor was proposed as the binding
TABLE 11-1 -- Classification of opioid compounds
|
Naturally occurring |
|
Morphine |
|
Codeine |
|
Papaverine |
|
Thebaine |
|
Semisynthetic |
|
Heroin |
|
Dihydromorphone/morphinone |
|
Thebaine derivatives (e.g., etorphine, buprenorphine) |
|
Synthetic |
|
Morphinan series (e.g., levorphanol, butorphanol) |
|
Diphenylpropylamine series (e.g., methadone) |
|
Benzomorphan series (e.g., pentazocine) |
|
Phenylapiperidine series (e.g., meperidine, fentanyl,
sufentanil, alfentanil, remifentanil) |
|
From Bailey PL, Egan TD, Stanley TH: Intravenous opioid
anesthetics. In Miller RD (ed): Anesthesia, 5th
ed. New York, Churchill Livingstone, 2000, p 276. |
 Figure 11-1
Chemical structures of piperidine and phenylpiperidine
analgesics. (From Gutstein HB, Akil H: Opioid analgesics. In
Hardman JG, Limbird LE [eds]: Goodman and Gilman's The Pharmacological Basis of
Therapeutics, 10th ed. New York, McGraw-Hill, 2001, pp 569–619.)
Figure 11-1
Chemical structures of piperidine and phenylpiperidine
analgesics. (From Gutstein HB, Akil H: Opioid analgesics. In
Hardman JG, Limbird LE [eds]: Goodman and Gilman's The Pharmacological Basis of
Therapeutics, 10th ed. New York, McGraw-Hill, 2001, pp 569–619.)
site for β-endorphin in the rat vas deferens. Pharmacologic actions of opioids
and the involved receptors have been analyzed ( Table
11-3
).
Biochemical studies attempted to purify the opioid receptor protein
but have not been successful. Since the early 1990s, molecular biological studies
have elucidated the molecular structures and signal transduction mechanisms of the
opioid receptors. Four different types of cDNA have been isolated as the members
of the opioid receptor family.[2]
It was demonstrated
that three of them correspond to the pharmacologically defined μ-, δ, and
κ-opioid receptors. The fourth receptor did not bind with opioid ligands with
high affinities. Later, a novel peptide called nociceptin, or orphanin FQ (see later),
was identified as an endogenous agonist of the fourth member of the opioid receptor
family.[3]
[4]
The characteristics of three opioid receptors and the nociceptin/orphanin FQ receptor
are listed in Table 11-4
.
The μ-receptors are located in both the brain and the spinal
cord[5]
and are thought to mediate a variety of
pharmacologic effects of opioids. Further pharmacologic classification of the μ-receptor
as μ1
, μ2
, and μ3
has been proposed. It
may be possible that posttranslational modification of the μ-receptor is the molecular
basis of μ-receptor subtypes. However, the molecular identity of these receptors
remains to be clarified.
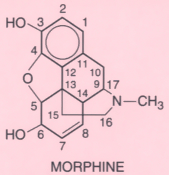
TABLE 11-2 -- Structures of opioids and opioid antagonists chemically related to morphine
|
|
|
Chemical Radicals and Position
*
|
|
|
Nonproprietary Name |
3 |
6 |
17 |
Other Changes
†
|
|
Morphine |
-OH |
-OH |
-CH3
|
— |
|
Heroin |
-OCOCH3
|
-OCOCH3
|
-CH3
|
— |
|
Hydromorphone |
-OH |
=O |
-CH3
|
(1) |
|
Oxymorphone |
-OH |
=O |
-CH3
|
(1), (2) |
|
Levorphanol |
-OH |
-H |
-CH3
|
(1), (3) |
|
Levallorphan |
-OH |
-H |
-CH2
CH=CH2
|
(1), (3) |
|
Codeine |
-OCH3
|
-OH |
-CH3
|
— |
|
Hydrocodone |
-OCH3
|
=O |
-CH3
|
(1) |
|
Oxycodone |
-OCH3
|
=O |
-CH3
|
(1), (2) |
|
Nalmefene |
-OH |
=CH2
|
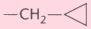
|
(1), (2) |
|
Nalorphine |
-OH |
-OH |
-CH2
CH=CH2
|
— |
|
Naloxone |
-OH |
=O |
-CH2
CH=CH2
|
(1), (2) |
|
Naltrexone |
-OH |
=O |
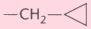
|
(1), (2) |
|
Buprenorphine |
-OH |
-OCH3
|
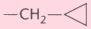
|
(1), (4) |
|
Butorphanol |
-OH |
-H |
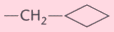
|
(1), (2), (3) |
|
Nalbuphine |
-OH |
-OH |
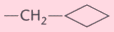
|
(1), (2) |
|
From Gutstein HB, Akil H: Opioid analgesics.
In Hardman JG, Limbird LE (eds): Goodman and Gilman's
The Pharmacological Basis of Therapeutics, 10th ed. New York, McGraw-Hill, 2001,
pp 569–619. |
*The
numbers 3, 6, and 17 refer to positions in the morphine molecule, as shown above.
†Other
changes in the morphine molecule are as follows:
- Single instead of double bond between C7 and C8.
- OH added to C14.
- No oxygen between C4 and C5.
- Endoetheno bridge between C6 and C14; 1-hydroxy-1,2,2-trimethylpropyl substitution
on C7.
Hydropathy analysis of the primary structures of the opioid receptors
indicates that opioid receptors possess seven transmembrane domains ( Fig.
11-2
). This is a characteristic structural feature of the G protein-coupled
receptor. The μ-, δ-, and κ-opioid receptors and the nociceptin receptor
share ∼50% amino acid sequence homology with each other.
 Figure 11-1
Chemical structures of piperidine and phenylpiperidine
analgesics. (From Gutstein HB, Akil H: Opioid analgesics. In
Hardman JG, Limbird LE [eds]: Goodman and Gilman's The Pharmacological Basis of
Therapeutics, 10th ed. New York, McGraw-Hill, 2001, pp 569–619.)
Figure 11-1
Chemical structures of piperidine and phenylpiperidine
analgesics. (From Gutstein HB, Akil H: Opioid analgesics. In
Hardman JG, Limbird LE [eds]: Goodman and Gilman's The Pharmacological Basis of
Therapeutics, 10th ed. New York, McGraw-Hill, 2001, pp 569–619.)