|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Waterton suggested the use of his woraria for the treatment of tetanus and rabies, and the few successful trials of the small samples of curare that were available then were used to treat these conditions.[331] For the next 175 years in Europe and North America, the drug remained a curiosity with no definite purpose. The medical community
 Figure 1-12
Mechanism of curare poisoning. Benjamin Brodie (A)
and Charles Waterton (B) collaborated in demonstrating
that animals could survive curare poisoning if artificial ventilation was provided.
(Courtesy of the National Library of Medicine, Bethesda, MD.)
Figure 1-12
Mechanism of curare poisoning. Benjamin Brodie (A)
and Charles Waterton (B) collaborated in demonstrating
that animals could survive curare poisoning if artificial ventilation was provided.
(Courtesy of the National Library of Medicine, Bethesda, MD.)
Thus far I have used solution of curarine up to 2% by intramuscular injection. With this dose, closure of the abdominal wall was achieved readily. There is not sufficient curare available, so I have not yet been able to ascertain the correct dose for this purpose.
The active principle was eventually found in several plant species, primarily Chondodendron tomentosum and Strychnos toxifera (see Fig. 1-9C ). King[333] isolated the active compound from the Chondodendron species that he called D-tubocurarine in 1935.
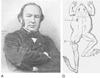 Figure 1-13
A, Claude Bernard (1813–1878)
was one of the greatest physiologists of all time and made several contributions
to the anesthesia literature. Bernard was born in Saint Julien, France, and was
educated in Paris. After his medical training, he became an assistant to François
Magendie, the leading physiologist of that era. He never practiced medicine, but
his research contributions pervade every field of modern medicine. In 1855, he succeeded
Magendie as Professor of Physiology at the College de France. A special Chair of
General Physiology was created for him at the Sorbonne. B,
Experiment by Claude Bernard, illustrating the site of action of curare at the junction
of the nerve and muscle. A ligature prevented injected curare (I) from reaching
the muscle of the frog's hind limb. After curare injection systemically, Bernard
observed that the limb contracted in response to a neural stimulus (N) applied above
the ligature. The opposite limb would contract only to direct electric stimulation
of the muscle but not to a neural stimulus. He showed that the nerve and muscle
were unaffected but that the connection between the nerve and the muscle was blocked.
[329]
(Courtesy of the National Library
of Medicine, Bethesda, MD.)
Figure 1-13
A, Claude Bernard (1813–1878)
was one of the greatest physiologists of all time and made several contributions
to the anesthesia literature. Bernard was born in Saint Julien, France, and was
educated in Paris. After his medical training, he became an assistant to François
Magendie, the leading physiologist of that era. He never practiced medicine, but
his research contributions pervade every field of modern medicine. In 1855, he succeeded
Magendie as Professor of Physiology at the College de France. A special Chair of
General Physiology was created for him at the Sorbonne. B,
Experiment by Claude Bernard, illustrating the site of action of curare at the junction
of the nerve and muscle. A ligature prevented injected curare (I) from reaching
the muscle of the frog's hind limb. After curare injection systemically, Bernard
observed that the limb contracted in response to a neural stimulus (N) applied above
the ligature. The opposite limb would contract only to direct electric stimulation
of the muscle but not to a neural stimulus. He showed that the nerve and muscle
were unaffected but that the connection between the nerve and the muscle was blocked.
[329]
(Courtesy of the National Library
of Medicine, Bethesda, MD.)
The introduction of curare into clinical practice was initiated by several individuals, beginning with Richard C. Gill[334] (1901–1958), whose contributions, like those of Waterton, arose out of a strong sense of wanderlust, combined with an interest in primitive medicine. As a young man growing up in Washington, D. C., Gill was expected to follow his father and older brother into the practice of medicine. Instead, his degree in 1929 from Cornell University was in English, and his career as a teacher was brief. He took a position with a rubber company in Lima, Peru, but with the stock market crash of 1929, he consolidated his savings, and together with his wife Ruth, purchased 750 acres of land in the Ecuadorian jungle. By 1930, Richard and Ruth Gill were the proud owners of a
While returning to the United States in 1932 for a brief holiday, Gill fell from his horse and developed a neurologic syndrome, initially thought to be multiple sclerosis, that eventually progressed to a painful spastic disorder. His neurologist Walter Freeman advised him that the arrow poison from South America might help cure his painful spasms, but it was nearly impossible to obtain the drug. Procuring curare and other herbal remedies thereafter became the focus of Gill's life until his death in 1958 at age 57. Gill obtained funding from Sayre Merrill, a wealthy Massachusetts businessman, for an expedition to retrieve indigenous medicinal plants from the Ecuadorian jungle. The primary aim was to retrieve adequate amounts of the crude curare preparation to begin clinical trials with the drug in cases of spasticity. It is noteworthy that Gill was in a unique position to obtain large quantities of curare because the Indian "medicine men," who jealously guarded the secrets of making the poisonous darts, trusted him.
After 4 years of planning, Gill's neurologic condition had improved, and the expedition was launched. In 1938, Richard and Ruth Gill returned with 11 kg of a crude curare mixture and 75 other indigenous medicinal preparations obtained from the Indian shamans. Sadly for the Gills, there was no interest in their jungle remedies. Initially, even the curare failed to generate any interest. Freeman was acquainted with Abram E. Bennett, a psychiatrist at the University of Nebraska who had expressed interest in the drug. Bennett had the crude concoction standardized by the pharmacologist A. R. McIntyre but failed to find any prolonged benefit for it in cases of spasticity ( Fig. 1-14A ).
Bennett then turned his attention to the use of curare during Metrazol convulsive therapy, which at that time was thought to be therapeutic for depression and mania but was complicated by a high incidence of bone fractures
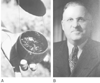 Figure 1-14
A, Richard Gill returned
from Ecuador in 1938 with 11 kg of a dark tarlike paste from which Squibb and Sons,
Inc., prepared the sterile, injectable solution, Intocostrin. B,
Harold Griffith and Enid Johnson (not shown) used Intocostrin in 43 cases of abdominal
surgery with success in 1942. (Courtesy of the Guedel Memorial Anesthesia
Center, San Francisco, CA.)
Figure 1-14
A, Richard Gill returned
from Ecuador in 1938 with 11 kg of a dark tarlike paste from which Squibb and Sons,
Inc., prepared the sterile, injectable solution, Intocostrin. B,
Harold Griffith and Enid Johnson (not shown) used Intocostrin in 43 cases of abdominal
surgery with success in 1942. (Courtesy of the Guedel Memorial Anesthesia
Center, San Francisco, CA.)
E. R. Squibb and Sons, together with their anesthesiologist consultant, Lewis H. Wright (1894–1974), were convinced that the true home for curare was with the anesthesiologist and in 1940, with some urging, Wright convinced Harold R. Griffith (1894–1985) (see Fig. 1-14B ) of Montreal, Canada to use the drug during general anesthesia when muscular relaxation was required by the surgeon. Previous attempts by Wright to generate interest in the drug had failed until Griffith agreed to use Intocostrin during cyclopropane anesthesia. Griffith and his junior colleague Enid Johnson reported the successful use of curare in 43 patients to provide muscular relaxation during surgical anesthesia in 1942.[337] They concluded that the agent provided relaxation without interfering with respiration. This was followed by a study in Iowa by Stuart Cullen[338] (1909–1979), who enthusiastically supported use of the drug to provide muscular relaxation without the need for deep levels of general anesthesia. T. Cecil Gray [339] (1913-) of Liverpool popularized the drug in England and developed an anesthetic technique consisting of profound muscular paralysis and nitrous oxide-oxygen anesthesia. Phyllis Harroun[340] [341] used high-dose curare, nitrous oxide, and morphine for thoracic surgery, thereby providing muscular relaxation without the use of flammable agents, a novel technique at the time. In 1947, William Neff[342] used nitrous oxide and oxygen supplemented with meperidine and curare and reported favorable anesthetic conditions. Although Griffith and Cullen had both maintained spontaneous
In a report from 1954, Henry K. Beecher (1907–1976) and D. P. Todd[343] concluded that the use of curare led to a nearly sixfold increase in postoperative complications and deaths. Similar cautionary articles followed their report but were refuted by others. Churchill-Davidson [344] advised in 1952 that a useful technique for monitoring the degree of neuromuscular blockade was to stimulate a peripheral nerve and observe the resulting muscular contraction. Specific devices designed to stimulate the ulnar nerve were promoted in the 1960s.[345] [346] Various modifications of the stimulus array have been described since that time to ensure complete return of neuromuscular function before allowing the patient to breathe unassisted.[347]
Since the introduction of curare into anesthesia practice, numerous additional neuromuscular blocking agents have been developed. Gallamine triethiodide was introduced in 1947, but like curare, it had undesirable effects on the autonomic nervous system, which prompted a search for other drugs.[348] Exploration of the structureactivity relationships of the plant alkaloids led to the development of the methonium compounds. In 1949, the neuromuscular blocking activity of one of this class of drugs, succinylcholine, was recognized,[349] and it was introduced clinically soon thereafter.[350] Succinylcholine and other similar agents were shown to depolarize the end plate, preventing additional motor contractions from activity in the motor nerve. Advances in chemistry allowed the synthesis of neuromuscular blocking agents that lacked the autonomic effects of curare. Since the discovery of curare, nearly 50 neuromuscular blocking agents have been introduced, but many of these drugs have been abandoned because of undesirable side effects. Drugs that have maintained favorable safety records are steroid-based synthetic neuromuscular blocking agents such as pancuronium (1966),[351] vecuronium (1980),[352] [353] and rocuronium (1991),[354] and they have replaced the older drugs, such as curare and gallamine, in the modern drug armamentarium.
|
|
|
|
|
|
|
|
|
|
|
|
|