|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The primary action of etomidate on the CNS is hypnosis, which is achieved in one arm-brain circulation after a normal induction dose (0.3 mg/kg). [583] [603] Etomidate has no analgesic activity. Plasma levels required during the maintenance of anesthesia are approximately 300 to 500 ng/mL, those required for sedation are 150 to 300 ng/mL, and those for awakening are 150 to 250 ng/mL (see Fig. 10-23 ).[24] [25] [597] [603] The mechanism by which etomidate produces hypnosis is not fully elucidated; however, it appears to be largely (but not solely) related to the GABA-adrenergic system. Its action may be antagonized by GABA antagonists.[604] In general, the mechanism of action of etomidate appears to be very similar to that established thus far for propofol (see earlier).[605] For etomidate it appears that the β2 - and β3 -subunits are more important for its hypnotic action than the GABAA α1 -subunit. [64] [606]
At a dose of 0.2 to 0.3 mg/kg, etomidate reduces CBF (by 34%) and CMRO2 (by 45%) without altering mean arterial pressure.[607] Thus, cerebral perfusion pressure is maintained or increased, and there is a beneficial net increase in the cerebral oxygen supply-demand ratio.[607] When given in doses sufficient to produce EEG burst suppression, etomidate acutely lowers ICP by up to 50% in patients with already increased ICP, and ICP returns to almost normal values.[608] [609] The decrease in ICP is maintained in the period immediately after intubation (also see Chapter 21 ).[609] To maintain the effects of etomidate on ICP, high infusion rates (60 µg/kg/min) are necessary.[610] In contrast to the situation with other neuroprotective agents such as thiopental, reduction of ICP and maintenance of burst suppression are not associated with a drop in mean arterial blood pressure. [609] Because cerebral vascular reactivity is still maintained after etomidate administration,[610] hyperventilation may theoretically further reduce ICP when used in conjunction with etomidate. In animals, etomidate has reduced brain disease after an acute cortical ischemic insult.[611] In 1993, Takanobu and coauthors [612] reported the neuroprotective qualities of etomidate to be equal to those of thiopental and superior to those of isoflurane in a rat model. Other investigators disagree on etomidate's neuroprotective qualities.[590] Deeper structures such as the brainstem may not be afforded ischemic protection by etomidate.[613]
A dose of 0.3 mg/kg rapidly reduces intraocular pressure by 30% to 60%.[614] The decrease in intraocular pressure after a single dose lasts 5 minutes, but the reduction may be maintained by an infusion of 20 µg/kg/min (see Chapter 65 ).[615]
Etomidate produces changes in the EEG similar to those produced by the barbiturates.[616] There is an initial increase in alpha-wave amplitude with sharp beta bursts followed by mixed delta-theta waves, with delta-wave activity predominating before the onset of periodic burst suppression. [616] The absence of beta waves in the initial phase of induction with etomidate is the major difference in the EEG changes induced by thiopental.[616] Etomidate has been associated with grand mal seizures[617] [618] and has been shown to produce increased EEG activity in epileptogenic foci. This feature has proved useful for intraoperative mapping of seizure foci before surgical ablation.[618] [619] Etomidate is also associated with a high incidence of myoclonic movement.[586] [591] Myoclonus is not thought to be associated with seizure-like EEG activity.[616] Giving etomidate to unpremedicated patients caused an increase in EEG activity in 22% of patients versus 17% of those receiving thiopental.[620] The myoclonic movement is believed to result from activity either in the brainstem or in deep cerebral structures.[575]
The effect of etomidate on auditory evoked potentials is similar to that produced by the inhaled anesthetics, with a dose-dependent increase in latency and a decreasing amplitude of the early cortical components (Pa and Nb).[621] The amplitude and latency of upper limb cortical somatosensory evoked potentials are positively affected after 0.4 mg/kg etomidate, which could theoretically
Etomidate has minimal effect on ventilation. It does not induce
histamine release in either healthy patients or those with reactive airway disease.
[624]
The ventilatory response to carbon dioxide
is depressed by etomidate, but the ventilatory drive at any given carbon dioxide
tension is greater than that after an equipotent dose of methohexital.[350]
Similarly, the response to occlusion pressure is less depressed after etomidate
than after an equivalent dose of methohexital.[625]
Induction with etomidate produces a brief period of hyperventilation,[177]
[626]
sometimes followed by a similarly brief period
of apnea,[626]
which results in a slight (![]() 15%)
increase in PaCO2
but no change in PaO2
.
[177]
[178]
The
incidence of apnea is altered by premedication.[592]
[627]
Hiccups or coughing may accompany etomidate
induction, and the incidence is similar to that after methohexital induction.[583]
15%)
increase in PaCO2
but no change in PaO2
.
[177]
[178]
The
incidence of apnea is altered by premedication.[592]
[627]
Hiccups or coughing may accompany etomidate
induction, and the incidence is similar to that after methohexital induction.[583]
In laboratory models, etomidate appears to be as effective as propofol in relaxing precontracted tracheal rings, but less effective than propofol in preventing tracheal ring contraction by muscarinic agonists.[628]
Etomidate attenuates the vasorelaxant responses of the pulmonary artery to acetylcholine and bradykinin by inhibiting both nitric oxide- and EDHF-mediated components, probably by inhibiting the endothelial [Ca2+ ]i transient in response to receptor activation. These actions on pulmonary vascular tone are similar to those observed with ketamine and propofol.[629]
The minimal effect of etomidate on cardiovascular function sets it apart from other rapid-onset induction agents (see Table 10-2 ).[177] [178] [179] [180] [627] [630] An induction dose of 0.3 mg/kg of etomidate given to cardiac patients for noncardiac surgery results in almost no change in heart rate, mean arterial pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure, central venous pressure, stroke volume, cardiac index, and pulmonary and systemic vascular resistance.[178] A relatively large dose of etomidate, 0.45 mg/kg (which is 50% larger than a normal induction dose),[627] also produces minimal changes in cardiovascular parameters. In patients with ischemic heart disease or valvular disease,[178] [630] etomidate (0.3 mg/kg) produces similar minimal alterations in cardiovascular parameters. In patients with mitral or aortic valve disease, etomidate may produce greater changes in mean arterial pressure (an approximate 20% decrease)[177] [631] than in patients without cardiac valvular disease. In isolated strips of myocardial muscle taken from failing and nonfailing hearts, etomidate exerts a dose-dependent negative inotropic effect that is reversible by β-adrenergic stimulation. With clinically therapeutic concentrations of etomidate, the negative inotropic effects noted were minimal and probably of no clinical significance.[632] After induction (18 mg) and infusion (2.4 mg/min), etomidate produces a 50% decrease in myocardial blood flow and oxygen consumption and a 20% to 30% increase in coronary sinus blood oxygen saturation.[162] The myocardial oxygen supply-demand ratio is thus well maintained.[162] [180] It has minimal effect on the QT interval. [633]
The hemodynamic stability seen with etomidate may be due in part to its unique lack of effect on both the sympathetic nervous system and baroreceptor function.[198] However, etomidate, which lacks analgesic efficacy, may not totally ablate the sympathetic response to laryngoscopy and intubation.[588] [591] For the least hemodynamic perturbation through the induction/intubation sequence, a low dose (1.5 to 5.0 µg/kg) of fentanyl is often combined with etomidate. [631] [634]
In a small study of 30 patients undergoing vascular procedures, etomidate (versus thiopental) resulted in inhibition of platelet function (increased bleeding time and inhibition of adenosine diphosphate and collagen-induced platelet aggregation) with increased blood loss.[635]
The concern surrounding the endocrine effects of etomidate stems from a letter by Ledingham and colleagues[636] in 1983 concerning ICU patients receiving long-term sedative infusions of etomidate while being mechanically ventilated for 5 days or longer. These investigators noted that in this subset of mechanically ventilated, multiple-trauma patients, the mortality rate was higher for 1981 to 1982 than in similar patients treated during 1979 to 1980. The earlier group had received primarily morphine and benzodiazepines for sedation, whereas patients in 1981 to 1982 had received primarily etomidate for sedation. It was postulated that adrenocortical suppression secondary to long-term etomidate infusion was the cause of the increased mortality.[577] Another ICU with similar patients receiving etomidate did not note increased mortality; these patients received high-dose steroids as part of the trauma protocol.[636] This finding helped confirm that hypothesis.
The specific endocrine effects manifested by etomidate are a dose-dependent reversible inhibition of the enzyme 11β-hydroxylase, which converts 11-deoxycortisol to cortisol, and a relatively minor effect on 17α-hydroxylase ( Fig. 10-24 ).[637] [638] These effects result in an increase in the cortisol precursors 11-deoxycortisol and 17-hydroxyprogesterone, as well as an increase in ACTH. The blockade of 11β-hydroxylase and, to a lesser extent, 17α-hydroxylase[637] appears to be related to the free imidazole radical of etomidate-binding cytochrome P450.[638] [639] [640] Such blockade results in inhibition of ascorbic acid resynthesis, which is required for steroid production in humans.[639] [640] Blockade of the cytochrome P450-dependent enzyme 11β-hydroxylase also results in decreased mineralocorticoid production and an increase in intermediaries (11-deoxycorticosterone).[338] [578] [580] Vitamin C supplementation restores cortisol levels to normal after the use of etomidate. [640] Because minor adrenocortical suppressive effects were shown to follow even single bolus doses,[578] [637] [641] concern about the use of etomidate for induction of anesthesia arose.[579] No large prospective studies have been conducted, but several smaller studies
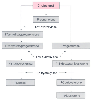 Figure 10-24
Pathway for the biosynthesis of cortisol and aldosterone.
The sites at which etomidate affects cortisol-aldosterone synthesis by its action
on 11β-hydroxylase (major site) and 17α-hydroxylase (minor site) are illustrated.
Figure 10-24
Pathway for the biosynthesis of cortisol and aldosterone.
The sites at which etomidate affects cortisol-aldosterone synthesis by its action
on 11β-hydroxylase (major site) and 17α-hydroxylase (minor site) are illustrated.
Duthie and associates[642] demonstrated that in otherwise healthy patients undergoing minor peripheral surgery, plasma cortisol levels were slightly depressed from preinduction levels for up to 1 hour postoperatively. The nadir of mean cortisol levels did not fall out of the normal range. 11-Deoxycorticosterone, substrate for the etomidate-inhibited 11β-hydroxylase, peaked at very high levels when compared with the thiopental control group.[642] In another study, orthopedic surgical patients underwent etomidate induction followed by an infusion of etomidate (average total dose, 68 mg). Temporary adrenocortical suppression, as measured by a reduced response to ACTH stimulation, was documented for 6 hours postoperatively and returned to normal by 20 hours postoperatively. Postoperative cortisol levels in the etomidate study patients were not significantly different from those in a group that received midazolam induction. As in the study of Duthie and coworkers,[642] mean cortisol levels in the etomidate group remained in the normal range at all times postoperatively. [643] Other studies have shown similar results when evaluating etomidate induction doses; none reported adverse outcomes secondary to short-term adrenocortical suppression.[338] [578] [637] [641] [642] [643] [644]
However, in each of the prospective etomidate studies documenting adrenocortical suppression without associated clinical sequelae, a conclusion of safety was not forthcoming. The reason was that these studies did not address high-stress procedures, in which the benefit of a high cortisol level in response to major stress could be desirable and etomidate's blockade of the response to ACTH could be detrimental. As part of a quality assurance program, we addressed this issue with a small, retrospective analysis of etomidate induction for high-stress procedures (vascular, thoracic, major intra-abdominal, and major retroperitoneal surgery) in 1993. Indices of adrenocortical function and perioperative outcome in patients who received induction doses of etomidate were compared with those in a control group that received thiopental. The incidence of perioperative wound infection, sepsis, miscellaneous infection, myocardial infarction, and hypotension and the need for perioperative vasopressor/inotropic support were evaluated, along with postoperative serum sodium levels. No difference was found between patients receiving etomidate and those receiving other induction agents for these high-stress procedures. In 1994, cortisol levels during and after coronary artery bypass surgery were compared in patients receiving total intravenous anesthesia with etomidate/fentanyl (mean etomidate dose of 87 ± 3 mg) versus midazolam/fentanyl. Except for the first hour after induction, cortisol levels were the same or higher in the etomidate group than in the midazolam group, a finding implying that the body's ability to respond to high surgical stress was still intact despite relatively high doses of etomidate. This study is further proof that etomidate is probably safe for use in major surgery.[645]
In summary, three facts suggest that the issue of temporary adrenocortical suppression after induction doses of etomidate is not clinically significant: (1) there are no known reports of any negative clinical outcome associated with etomidate induction despite millions of uses; (2) after etomidate induction, the nadir of cortisol levels usually remains in the low-normal range, and the adrenocortical suppression is a relatively short-lived phenomenon; and (3) high-stress surgery can overcome the temporary adrenocortical suppression caused by etomidate.
Although etomidate provides stable hemodynamics and minimal respiratory depression, it is associated with several adverse effects when used for induction, including nausea and vomiting, pain on injection, myoclonic movement, and hiccups. [575] [583] [586] [590] [591] [592] [593] Etomidate has been associated with a high (30% to 40%) incidence of nausea and vomiting.[586] [591] [592] [593] [594] In contrast, an incidence of 10% to 20% is reported with methohexital[583] [592] and thiopental[591] [646] but some studies have shown no difference.[587] [591] More recently, etomidate in a lipid emulsion was associated with an incidence of postoperative nausea equivalent to that of propofol. [647] The addition of fentanyl to etomidate further increases the incidence of nausea and vomiting.[586] [591] Nausea and vomiting are the most common reasons for patients to rate anesthesia with etomidate unsatisfactory.[593] It seems prudent to avoid etomidate in patients predisposed to nausea and vomiting.
Superficial thrombophlebitis of the vein used may occur 48 to 72 hours after etomidate injection.[648] The incidence may be as high as 20% when etomidate is given alone through a small (21-gauge) intravenous needle. Intra-arterial injection of etomidate is not associated with local or vascular disease.[575] Pain on injection, similar in incidence to that with propofol,[275] can be essentially eliminated by injecting lidocaine immediately before the
The incidence of muscle movement (myoclonus) and hiccups is also highly variable (0% to 70%), but myoclonus is reduced by premedication with either a narcotic or a benzodiazepine.[594] Both fast and slow injection techniques have also been advocated for reducing myoclonus.[590] [594]
Etomidate enhances the neuromuscular blockade of nondepolarizing neuromuscular blockers.[651] [652] Hepatic function is unaltered by etomidate.[583] [598] In vitro, etomidate inhibits aminolevulinic acid synthetase, but it has been administered to patients with porphyria without inducing an acute attack of porphyria.[646]
The carrier for etomidate, propylene glycol, has also been reported to have some negative effects. Some reports suggest that propylene glycol can be associated with a small degree of hemolysis.[584] Additionally, high-dose prolonged infusion has been reported to result in propylene glycol toxicity (a hyperosmolar state).[653]
|
|
|
|
|
|
|
|
|
|
|
|
|