Pharmacokinetics
The pharmacokinetics of etomidate has been calculated after single
bolus doses and after continuous infusion (see Table
10-1
).[24]
[25]
[26]
[27]
[598]
The time course of plasma disappearance after a 0.3-mg/kg bolus is shown in Figure
10-23
. The kinetics of etomidate is best described by an open three-compartment
model.[26]
[27]
[598]
The drug has an initial distribution half-life
of 2.7 minutes, a redistribution half-life of 29 minutes,[27]
[598]
and an elimination half-life that varies from
2.9 to 5.3 hours.[24]
[25]
[26]
[27]
[598]
Clearance of etomidate by the liver is high (18 to 25 mL/kg/min), with a hepatic
extraction ratio of 0.5 ± 0.9.[24]
[26]
[27]
[583]
[598]
Thus, drugs affecting hepatic blood flow
alter
its elimination
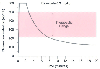 Figure 10-23
Simulated time course of plasma levels of etomidate after
an induction dose of 0.3 mg/kg. Plasma levels required for hypnosis during surgery
are 300 to 500 ng/mL, with awakening usually occurring at levels lower than 225 ng/mL.
Figure 10-23
Simulated time course of plasma levels of etomidate after
an induction dose of 0.3 mg/kg. Plasma levels required for hypnosis during surgery
are 300 to 500 ng/mL, with awakening usually occurring at levels lower than 225 ng/mL.
half-life. Because redistribution is the mechanism whereby the effect of a bolus
of etomidate is dissipated, hepatic dysfunction should not appreciably alter recovery
from its hypnotic effect. The volume of distribution at steady state is 2.5 to 4.5
L/kg.[24]
[25]
[26]
[27]
[598]
Etomidate
is 75% protein bound.[599]
Pathologic conditions
that alter serum proteins (e.g., hepatic or renal disease) vary the amount of the
free (unbound) fraction and may cause a given dose to have an exaggerated pharmacodynamic
effect.[599]
A hemorrhagic shock model in pigs
bled to a mean pressure of 50 mm Hg did not alter etomidate's pharmacokinetics or
pharmacodynamics.[600]
This finding contrasts with
the marked changes seen in this same model with other intravenous anesthetics.
In patients with cirrhosis, the volume of distribution is doubled,
but clearance is normal; the result is an elimination half-life that is twice normal.
[601]
It is likely that the initial distribution
half-life and clinical effect are unchanged. Increasing age is associated with a
smaller initial volume of distribution and decreased clearance of etomidate.[602]
The relatively short elimination half-life and the rapid clearance of etomidate
make it suitable for administration in a single dose, in multiple doses, or in a
continuous infusion.
 Figure 10-23
Simulated time course of plasma levels of etomidate after
an induction dose of 0.3 mg/kg. Plasma levels required for hypnosis during surgery
are 300 to 500 ng/mL, with awakening usually occurring at levels lower than 225 ng/mL.
Figure 10-23
Simulated time course of plasma levels of etomidate after
an induction dose of 0.3 mg/kg. Plasma levels required for hypnosis during surgery
are 300 to 500 ng/mL, with awakening usually occurring at levels lower than 225 ng/mL.