|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tennant and colleagues[181] reported acute depression of serum folate concentrations in patients fed preoperatively with parenteral nutrition solutions. Their studies seem to indicate that the amino acid component of the solutions may be at least partially responsible for the fall in serum folate level, as suggested by Krebs and coworkers.[182] Tennant and colleagues[183] cautioned that further investigation may be indicated in patients receiving postoperative parenteral nutrition after anesthesia exposure to nitrous oxide, which may alter serum methionine and folate concentrations.[184] [185]
| Infectious Complications | Technical Complications |
|---|---|
| Insertion-site contamination | Pneumothorax |
| Contamination during insertion | Tension pneumothorax |
| Contamination during routine care | Hemothorax |
| Catheter contamination | Hydrothorax |
| Improper technique of catheter insertion | Hydromediastinum |
| Administration of blood through feeding catheter | Cardiac tamponade |
| Use of catheter to measure central venous pressure | Brachial plexus injury |
| Use of catheter to obtain blood samples | Horner's syndrome |
| Use of catheter to administer medications | Phrenic nerve paralysis |
| Contaminated solution during preparation of additives | Carotid artery injury |
| Contaminated tubing through connections | Subclavian artery injury |
| Three-way stopcocks in system | Subclavian hematoma |
| Secondary contamination | Thrombosis, subclavian vein or superior vena cava |
| Septicemia, bacterial or fungal | Arteriovenous fistula |
| Septic emboli | Venobronchial fistula |
| Osteomyelitis of clavicle | Air embolism |
| Septic arthritis | Catheter embolism |
| Endocarditis | Thromboembolism |
|
|
Catheter misplacement |
|
|
Cardiac perforation |
|
|
Endocarditis |
|
|
Thoracic duct laceration |
|
|
Innominate or subclavian vein laceration |
| From Dudrick SJ: Parenteral nutrition. In Dudrick SJ, Baue AE, Eiseman B, et al (eds): Manual of Preoperative and Postoperative Care. Philadelphia, WB Saunders, 1983, p 86. | |
The most common errors are underestimating phosphate needs (40 to 100 mEq/day) and overestimating calcium requirements (3 to 8 mEq/day). The most common abnormality in patients receiving TPN is a mild-to-moderate elevation in results of liver function tests. This may be a result of fatty infiltration of the liver that occurs with excess glucose administration. In a patient who begins with normal indexes of liver function, twofold elevations in serum glutamic oxaloacetate (SGOT) and serum glutamic pyruvate transaminase (SGPT) levels and a progressive mild rise in alkaline phosphatase level are not uncommon. If an increase in bilirubin occurs and SGOT and SGPT levels continue to rise, a cause other than the TPN infusion should be sought. Unfortunately, maintenance or restoration of lean body mass in hypercatabolic patients can be difficult because of the inevitable increased breakdown of muscle protein supplying amino acids for gluconeogenesis. An attempt to induce anabolism by infusing large amounts of carbohydrate increases the metabolic rate, the conversion of carbohydrate into fat, and the rate of DVCO2 ; it does not promote increased protein synthesis, and it results in self-defeating hypermetabolism.[186] Hypercatabolic patients must be given adequate amino acids to achieve nitrogen equilibrium. Patients with persistent negative nitrogen balance should have the ratio of nitrogen to calories increased without increasing nonprotein calories beyond their use capabilities.[153] [186] [187]
| Complications | Etiology |
|---|---|
| Glucose Metabolism | |
| Hyperglycemic glycosuria, osmotic diuresis, nonketotic hyperosmolar dehydration, and coma | Excessive total dose or rate of infusion of dextrose; inadequate endogenous insulin; glucocorticoids; sepsis |
| Ketoacidosis of diabetes mellitus | Inadequate endogenous insulin response; inadequate exogenous insulin therapy |
| Postinfusion (rebound) hypoglycemia | Persistence of endogenous insulin production secondary to prolonged stimulation of islet cells by high-carbohydrate infusion |
| Respiratory acidosis with hypercarbia | Excessive dextrose administration |
| Amino Acid Metabolism | |
| Hyperchloremic metabolic acidosis | Excessive chloride and monohydrochloride content of crystalline amino acid solutions |
| Serum amino acid imbalance | Unphysiologic amino acid profile of the nutrient solution; different amino acid use with various disorders |
| Hyperammonemia | Excessive ammonia in protein hydrolysate solutions: deficiencies of arginine, ornithine, aspartic acid, and/or glutamic acid in crystalline amino acid solutions; primary hepatic disorder |
| Prerenal azotemia | Excessive total dose or rate of infusion of protein hydrolysate or amino acid solutions |
| Lipid Metabolism | |
| Hyperlipidemia | Excessive total dose or rate of administration of fat emulsion |
| Hyperamylasemia | Lipoid pancreatitis |
| Hyperbilirubinemia | Excessive dose or rate of administration of fat emulsion |
| Hypoxia | Interstitial lipoid pneumonitis with alveolar-capillary block |
| Serum deficiencies of phospholipid linoleic and arachidonic acids; serum elevations of 5.8.11-eicosatrienoic acid | Inadequate essential fatty acid administration; inadequate vitamin E administration |
| Calcium and Phosphorus Metabolism | |
| Hypophosphatemia Decreased erythrocyte 2,3-diphosphoglycerate | Inadequate phosphorus administration; redistribution of serum phosphorus into cells and/or bone |
| Increased affinity of hemoglobin for oxygen |
|
| Aberrations of erythrocyte intermediary metabolites |
|
| Hypocalcemia | Inadequate calcium administration; reciprocal response to phosphorus repletion without simultaneous calcium infusion; hypoalbuminemia |
| Hypercalcemia | Excessive calcium administration with or without high doses of albumin; excessive vitamin D administration |
| Vitamin D deficiency; hypervitaminosis D | Inadequate or excessive vitamin D administration |
| Miscellaneous Complications | |
| Hypokalemia | Inadequate potassium intake relative to increased requirements for protein anabolism: diuresis |
| Hyperkalemia | Excessive potassium administration, especially in metabolic acidosis, renal decompensation |
| Hypomagnesemia | Inadequate magnesium administration relative to increased requirements for protein anabolism and glucose metabolism |
| Hypermagnesemia | Excessive magnesium administration; renal decompensation |
| Anemia | Iron deficiency; folic acid deficiency; vitamin B12 deficiency; copper deficiency; other deficiencies |
| Bleeding | Vitamin K deficiency |
| Hypervitaminosis A | Excessive vitamin A administration |
| Elevations in SGOT, SGPT, and serum alkaline phosphatase | Enzyme induction caused by amino acid imbalance; excessive glycogen and/or fat deposition in the liver |
| Cholestatic hepatitis | Decreased water content of bile; amino acid and/or fatty acid imbalance |
| From Dudrick SJ: Parenteral nutrition. In Dudrick SJ, Baue AE, Eiseman B, et al (eds): Manual of Preoperative and Postoperative Care. Philadelphia, WB Saunders, 1983, p 86. | |
In 1976, Doekel and coworkers[188] reported that normal volunteers fed 400 kcal/day of carbohydrate orally for 10 days experienced a decrease in ventilatory response to hypoxia, a measure of respiratory drive (see Fig. 77-10 ). Weissman and associates[189] observed that 7 days of infusion of 400 kcal/day of 5% dextrose resulted in a reduced mean inspiratory flow, another measure of neuromuscular drive.
Arora and Rochester[190] found that chronically ill, nutritionally depleted patients had significant reductions in respiratory muscle strength, as measured by maximal inspiratory and expiratory pressures; endurance, as measured by maximal voluntary ventilation; and vital capacity. High-protein nutritional support improves respiratory muscle strength and ventilatory drive.[152] [191]
Excessive glucose administration leads to an increase in minute ventilation that is proportional to an increase in DVCO2 caused by oxidation of carbohydrate (RQ = 1) or lipogenesis (RQ = 8).[192] Infusion of glucose as a sole energy source can lead to increased respiratory work because ventilation increases in response to added CO2 .
In summary, Weismann and Askanazi[192] recommended that nonprotein calories be administered as 50% carbohydrate and 50% lipid. They recommended this balanced supply of energy sources for patients with poor respiratory function and for those who are septic and hypermetabolic. This approach leads to less DVCO2 than occurs with methods supplying 100% of the nonprotein calories as glucose. The combined substrate technique causes less increase in energy expenditure and norepinephrine secretion in the septic and hypermetabolic patient[193] and prevents essential fatty acid deficiency.[150] Later work suggests that using glucose-based formula but reducing the total calories to that which the patient is actually consuming reduces the complications of overfeeding and hyperlipidemia. Essential fatty acids must be replaced with 500 mL of lipid three times per week. Hyperglycemia should be aggressively treated with insulin to improve outcomes. Long-term propofol infusions provide significant lipid calories, and nutritional support must be adjusted.[194]
The most common cause of hypoglycemia is a slowing or a cessation of infusion. The increased secretion of insulin that accompanies TPN when suddenly unopposed by exogenous glucose results in hypoglycemia. For this reason, TPN solutions should never be abruptly discontinued in the operating room without being replaced with a concentrated glucose solution, such as 10% dextrose solution.
Extended TPN using lipid emulsions may injure the bilirubin-transfer mechanism in the liver and lead to progressive cholestasis. This is usually a benign, readily reversible state, but continued TPN without a change in the constituents can cause liver injury with hepatocyte necrosis and periportal fibrous changes. In a study by Allardyce,[195] patients with a high lipid intake had significant elevations of the serum bilirubin and alkaline phosphate levels at the completion of parenteral nutrition. Cholestatic jaundice is associated with administration of lipid emulsion in a dose of 3 g/kg/day in patients fed intravenously for more than 3 weeks. The condition improves, with a return of liver function to normal, when the dose of lipid emulsion is reduced or when intravenous feeding is discontinued. Lipid emulsion may be limited to 1 g/kg/day, and liver function may be evaluated twice weekly. It appears that the dose of lipid emulsion should be reduced if a progressive rise in serum alkaline phosphatase level occurs. Early use of intravenous fat emulsion in trauma patients is associated with increased septic complications compared with glucose-based intravenous feedings.[162] [196]
Bistrian and colleagues[134] pointed out that the tendency to retain water is characteristic of many of the disease states for which TPN is employed, including postoperative states, shock resuscitation, congestive heart failure, oliguric renal failure, hepatic insufficiency, and severe malnutrition. The antinatriuresis that results from the hyperinsulinemia seen with glucose-based TPN can lead to serious water overload in a brief period. This can be minimized or avoided by matching total fluid losses plus 5 dL, by limiting sodium intakes to less than 40 mEq of sodium beyond loss replacement, and by limiting insulin response by the use of a mixed-fuel system.
Many patients who are candidates for enteral feeding are at increased risk for aspiration because of mechanical ventilatory support or altered consciousness. Trauma patients with pulmonary injuries such as contusions, aspiration at the scene, hemopneumothorax, or acute respiratory distress syndrome (ARDS) are at even greater risk of pulmonary aspiration of gastric contents. Advances in the field of tube feeding have made early enteral nutrition a valuable tool in initiating nutrition therapy through a feeding tube in the proximal jejunum. A feeding algorithm was developed by the American Society for Parenteral and Enteral Nutrition to assist in selecting the type and route of administration of nutritional support ( Fig. 77-22 ). Enteral nutrition may possess several advantages over parenteral nutrition for postoperative or post-trauma patients. In patients with a functional gastrointestinal tract, enteral feeding assists in maintaining a thicker gut barrier by nourishing the enterocytes at the local level, which protects the host from bacterial translocation from gut lumen to the blood. Jejunal tube feeding has advantages over gastric tube feeding, including faster metabolic recovery, less vomiting, and less risk of regurgitation and aspiration.
Enteral nutrition can be started at a rate of 10 to 40 mL/hour and increased by 20 mL every 8 hours to reach target rates over a period of 24 to 48 hours. Standard tube-feeding solutions provide 1 kcal/mL, whereas concentrated formulas provide 1.5 to 2 kcal/mL and contain a standard mix of vitamins and minerals that meet recommended daily allowance when 1700 to 2000 mL of solution are provided. Supplements such as glutathione, vitamin E, and β-carotene are important for providing food antioxidants. Additional supplements are needed
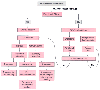 Figure 77-22
Clinical decision algorithm governing assessments for
route of nutrition support in adults. GI, gastrointestinal; PN, parenteral nutrition.
(Data from Aspen Board of Directors: Clinical Pathways and Algorithms for
Delivery of Parenteral and Enteral Nutrition Support in Adults. Silver Spring, MD,
American Society for Parenteral and Enteral Nutrition, 1998.)
Figure 77-22
Clinical decision algorithm governing assessments for
route of nutrition support in adults. GI, gastrointestinal; PN, parenteral nutrition.
(Data from Aspen Board of Directors: Clinical Pathways and Algorithms for
Delivery of Parenteral and Enteral Nutrition Support in Adults. Silver Spring, MD,
American Society for Parenteral and Enteral Nutrition, 1998.)
|
|
|
|
|
|
|
|
|
|
|
|
|