GLUCOSE HOMEOSTASIS DURING PARENTERAL NUTRITION
Normally, the concentration of plasma glucose is kept within close
limits. There is a constant requirement for glucose by glucose-dependent tissues
(e.g., brain, erythrocytes). Equally, there are detrimental effects of extreme hyperglycemia
(e.g., hyperosmolar coma).[16]
[17]
The mechanisms responsible for the regulation of plasma glucose levels are of fundamental
importance to the entire metabolic response to hyperalimentation.[18]
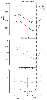 Figure 77-10
Effects of semistarvation on metabolic rate and hypoxic
and hypercapnic ventilatory responses. The hypoxic response is quantitated by A,
defined in the equation VE = Vo
+ A/(PaO2
− 32),
in which VE is minute ventilation, PaO2
is alveolar oxygen tension, and Vo
is the asymptote for ventilation obtained
by extrapolation. The hypercapnic response is quantitated by S, defined in the equation
VE = S(PaCO2
− B), in which PaCO2
is end-tidal CO2
tension, and B is the extrapolated intercept on the abscissa
(PaCO2
axis). ψO2
and ψCO2
declined, reaching the lowest
level by the 10th day, as did the hypoxic response. The hypercapnic response declined
slightly but not significantly. The asterisk denotes
a significant difference from the control (P <
.05), and brackets indicate ± SEM. (Adapted from Doekel RC Jr, Zwillich
CW, Scoggin CH, et al: Clinical semi-starvation: Depression of hypoxic ventilatory
response. N Engl J Med 295:358, 1976.)
Figure 77-10
Effects of semistarvation on metabolic rate and hypoxic
and hypercapnic ventilatory responses. The hypoxic response is quantitated by A,
defined in the equation VE = Vo
+ A/(PaO2
− 32),
in which VE is minute ventilation, PaO2
is alveolar oxygen tension, and Vo
is the asymptote for ventilation obtained
by extrapolation. The hypercapnic response is quantitated by S, defined in the equation
VE = S(PaCO2
− B), in which PaCO2
is end-tidal CO2
tension, and B is the extrapolated intercept on the abscissa
(PaCO2
axis). ψO2
and ψCO2
declined, reaching the lowest
level by the 10th day, as did the hypoxic response. The hypercapnic response declined
slightly but not significantly. The asterisk denotes
a significant difference from the control (P <
.05), and brackets indicate ± SEM. (Adapted from Doekel RC Jr, Zwillich
CW, Scoggin CH, et al: Clinical semi-starvation: Depression of hypoxic ventilatory
response. N Engl J Med 295:358, 1976.)
During intravenous infusion of glucose, two processes can minimize
changes in plasma glucose concentration: reduction in the rate of glucose production
and enhanced ability to clear glucose from the bloodstream. Wolfe and coworkers
[19]
demonstrated that when glucose was infused
into
normal volunteers at the rate of 1 mg/kg/min (84 mL/hour of 5% dextrose in water
in a 70-kg patient), endogenous glucose production was suppressed. One of the principal
reasons infused glucose is of nutritional benefit is that the suppression of glucose
production resulting from glucose infusion spares amino acids that would otherwise
be catabolized to provide gluconeogenic precursors. The rate of glucose infusion
that provides the maximal benefit depends on the condition of the patient.[20]
From a clinical point of view, the justification for administering
glucose at rates in excess of 3 to 4 mg/kg/min must therefore reside in the ability
of the body to oxidize the infused glucose. Table
77-3
shows that even during a 4-mg/kg/min infusion, less than one half
of the infused glucose was directly oxidized, and that at an infusion rate of 9 mg/kg/min,
approximately one third of the glucose was directly oxidized.[21]
At this high level of glucose infusion, however, only 61.6% of CO2
was
attributed to direct oxidation of glucose. Even at these levels of glucose supply,
other substrates are being oxidized.
The percentage of CO2
from glucose infusion also reaches
a plateau at around 60% in burn patients, regardless of the infusion rate.[22]
If insulin is infused simultaneously with glucose, the glucose clearance rate increases,
but the rate of glucose oxidation is unaltered. Perhaps 22% of carbon dioxide that
is coming from fat oxidation can be attributed to the conversion of excess infused
glucose to fat.
Hyperglycemia associated with insulin resistance is a common complication
observed in critically ill patients, even in those patients without a history of
diabetes.[23]
Van den Berghe and coworkers[24]
[25]
examined the role of intensive insulin therapy
(i.e., maintenance of blood glucose no higher than 110 mg/dL) to improve mortality
in critically ill patients compared with conventional therapy (i.e., insulin treatment
only when plasma glucose exceeds 215 mg/dL and maintenance of glycemia between 180
and 200 mg/dL). The mortality rate for the patients receiving intensive insulin
therapy was significantly lower than for the group assigned to receive conventional
therapy. The benefit was seen specifically in patients with stays of more than 5
days in the intensive care unit and was achieved principally through a reduction
in the incidence of multiple organ failure with an identifiable septic focus.
Intensive glycemic control also reduced the morbidity associated
with critical illness. Significantly fewer patients in the intensive-treatment group
required prolonged ventilatory support and renal replacement therapy, whereas the
proportion of patients needing inotropic or vasopressor therapy, or both, was the
same in the two groups. Overall, intensive insulin therapy reduced the length of
stay in the intensive care unit among patients requiring intensive care for more
than 5 days. The reason for improvement in intensive insulintreated patients remains
obscure until other investigators can replicate the improvement in mortality in critically
ill patients.
TABLE 77-3 -- Glucose oxidation during total parenteral nutrition in five postoperative
patients
|
Glucose Infusion Rate (mg/kg/min) |
Glucose Oxidation Rate (mg/kg/min) |
CO2
from Glucose
(%) |
Respiratory Quotient |
|
4.0 |
1.87 ± 0.29 |
51.1 ± 5.0 |
0.91 ± 0.03 |
|
7.0 |
2.46 ± 0.088 |
54.6 ± 3.69 |
1.13 ± 0.16 |
|
9.0 |
3.16 ± 0.68 |
61.6 ± 13.46 |
1.12 ± 0.05 |
|
From Wolfe RR, O'Donnell TF, Stone MD, et al: Investigation
of factors determining the optimal glucose infusion rate in total parenteral nutrition.
Metabolism 29:892, 1980. |
 Figure 77-10
Effects of semistarvation on metabolic rate and hypoxic
and hypercapnic ventilatory responses. The hypoxic response is quantitated by A,
defined in the equation VE = Vo
+ A/(PaO2
− 32),
in which VE is minute ventilation, PaO2
is alveolar oxygen tension, and Vo
is the asymptote for ventilation obtained
by extrapolation. The hypercapnic response is quantitated by S, defined in the equation
VE = S(PaCO2
− B), in which PaCO2
is end-tidal CO2
tension, and B is the extrapolated intercept on the abscissa
(PaCO2
axis). ψO2
and ψCO2
declined, reaching the lowest
level by the 10th day, as did the hypoxic response. The hypercapnic response declined
slightly but not significantly. The asterisk denotes
a significant difference from the control (P <
.05), and brackets indicate ± SEM. (Adapted from Doekel RC Jr, Zwillich
CW, Scoggin CH, et al: Clinical semi-starvation: Depression of hypoxic ventilatory
response. N Engl J Med 295:358, 1976.)
Figure 77-10
Effects of semistarvation on metabolic rate and hypoxic
and hypercapnic ventilatory responses. The hypoxic response is quantitated by A,
defined in the equation VE = Vo
+ A/(PaO2
− 32),
in which VE is minute ventilation, PaO2
is alveolar oxygen tension, and Vo
is the asymptote for ventilation obtained
by extrapolation. The hypercapnic response is quantitated by S, defined in the equation
VE = S(PaCO2
− B), in which PaCO2
is end-tidal CO2
tension, and B is the extrapolated intercept on the abscissa
(PaCO2
axis). ψO2
and ψCO2
declined, reaching the lowest
level by the 10th day, as did the hypoxic response. The hypercapnic response declined
slightly but not significantly. The asterisk denotes
a significant difference from the control (P <
.05), and brackets indicate ± SEM. (Adapted from Doekel RC Jr, Zwillich
CW, Scoggin CH, et al: Clinical semi-starvation: Depression of hypoxic ventilatory
response. N Engl J Med 295:358, 1976.)