RESPIRATORY FAILURE
Respiratory failure refers to the inadequate exchange of gas by
the cardiopulmonary system. In order to distinguish between the types of respiratory
failure it is useful to determine the difference between the alveolar partial pressure
of oxygen (PAO2
) and the arterial partial
pressure of oxygen (PaO2
), or P(A-a)O2
.
The PaCO2
is obtained from an arterial
blood gas, whereas the PAO2
is calculated
from the alveolar gas equation:
PAO2
= (PB
− PH2
O)FIO2
− PaCO2
/RQ
where PB is the barometric pressure, PH2
O
is the partial pressure of water in the alveoli (47 mm Hg at body temperature), FIO2
is the inspired fraction of oxygen, PaCO2
is the partial pressure of CO2
measured on an arterial blood gas, and
RQ is the respiratory quotient (usually assumed to be 0.8). A normal P(A-a)O2
when a person is breathing room air is less than 15 mm Hg and increases linearly
with age and FIO2
. In a person 80 years
of age breathing room air, a normal P(A-a)O2
is 25 mm Hg; when breathing an FIO2
of
1.0, a P(A-a)O2
of 60 is normal.[1]
Respiratory failure can be classified into three categories:
- Ventilatory failure, or a decrease in the
bulk flow of gas in and out of the lungs resulting in alveolar hypoventilation and
retention of CO2
. This rise in CO2
is frequently accompanied
by a drop in the PaO2
but with a normal
P(A-a)O2
.
- Hypoxic respiratory failure, or a significant
impairment in the molecular exchange of oxygen across the pulmonary alveolar-capillary
membrane, causing a decrease in PaO2
and
an increase in P(A-a)O2
.
- Combined ventilatory and hypoxic respiratory failure,
which manifests with a low PaO2
, an elevated
PaCO2
as well as an increased P(A-a)O2
.
[2]
Respiratory failure can thus be conceptualized
as having components of ventilatory pump failure (failure to adequately move
gases in and out of the pulmonary system) and/or lung failure ( Fig.
75-1
).
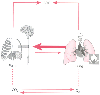 Figure 75-1
Schematic representation of the pathogenesis of acute
respiratory failure (ARF). The ventilatory pump is composed of the chest cage, ventilatory
muscles, and nervous system elements involved in respiration. The pump primarily
affects carbon dioxide excretion (CO2
). The lung involves the elements
that allow inspired gas to exchange with pulmonary blood flow and primarily affects
blood oxygenation (O2
). The large arrow from lung to pump represents
the finding that lung disease often increases the work of the pump.
Figure 75-1
Schematic representation of the pathogenesis of acute
respiratory failure (ARF). The ventilatory pump is composed of the chest cage, ventilatory
muscles, and nervous system elements involved in respiration. The pump primarily
affects carbon dioxide excretion (CO2
). The lung involves the elements
that allow inspired gas to exchange with pulmonary blood flow and primarily affects
blood oxygenation (O2
). The large arrow from lung to pump represents
the finding that lung disease often increases the work of the pump.
Ventilation is the bulk movement of gas into and out of the tracheobronchial
tree. Failure of the ventilatory pump can occur due to (1) fatigue of the ventilatory
muscles (e.g., in cardiopulmonary disease with increased dead-space ventilation),
(2) decreases in chest wall compliance (e.g., scoliosis, kyphosis, pain and splinting
after abdominal or thoracic surgery), (3) neuromuscular disease (e.g., muscular dystrophy,
Guillain-Barré syndrome, amyotrophic lateral sclerosis), and (4) central nervous
system (CNS) dysfunction (e.g., traumatic injury, pharmacologic depression, electrolyte
abnormality). Ventilatory failure frequently requires treatment with PPV to improve
the bulk movement of gas into and out of the pulmonary system and thus to facilitate
adequate ventilation and elimination of CO2
.
Hypoxemia manifests as a PaO2
of less than 60 mm Hg. Hypoxemia can be considered due to a combination of six factors
that can be grouped according to the P(A-a)O2
( Table 75-1
).[1]
Tissue oxygenation is primarily dependent on oxygen delivery. Oxygen is transported
from the alveoli to tissues in two forms: (1) oxygen that is dissolved in the blood
plasma, and (2) oxygen that is bound to hemoglobin (Hb) in the red blood cell. The
oxygen content of blood is the sum of the Hb-bound O2
and the dissolved
O2
. The Hb-bound O2
is a function of arterial oxygen saturation
(SaO2
), whereas the dissolved component
is a function of the arterial partial pressure of oxygen (PaO2
).
When fully saturated, 1.34 mL of oxygen is bound to each gram of Hb. Because oxygen
is not very
TABLE 75-1 -- Causes of hypoxemia
|
Causes |
P(A-a)O2
|
|
Decreased barometric pressure |
Normal |
|
Decreased FIO2
|
Normal |
|
Hypoventilation |
Normal |
Shunting (high  s/ s/ t) t) |
Increased |
|
Ventilation-perfusion mismatch |
Increased |
|
Diffusion impairment |
Increased |
 s/ s/ t, shunted blood flow/cardiac output ratio. t, shunted blood flow/cardiac output ratio. |
soluble in plasma, the dissolved component of blood oxygen is much less than the
Hb-bound component. For each mm Hg of arterial partial pressure of oxygen, 1 dL
of plasma will contain 0.003 mL of dissolved oxygen. The content of arterial oxygen
(CaO2
) can therefore be expanded to:
CaO2
= [(1.34)[Hb](SaO2
)]
+ [(PAO2
)(0.003)]
Oxygen delivery is the product of blood oxygen content and the
cardiac output. Hypoxia is clinically defined as inadequate oxygen delivery at the
tissue level, whereas hypoxemia refers to a deficiency of oxygen in arterial blood.
Thus, tissue hypoxia can result from hypoxemia or impaired perfusion. The physiologic
responses to tissue hypoxia are, primarily, to increase oxygen delivery by increasing
cardiac output and, secondarily, to increase ventilation. The primary goal of oxygen
therapy is to increase the alveolar oxygen content, thus increasing the alveolar
partial pressure of oxygen (PAO2
). When
hypoxemia results from a low PAO2
, increasing
the fraction of inspired oxygen (FIO2
)
will result in an increase in PaO2
. On
the other hand, when hypoxia results from ventilatory demands that exceed the ability
of the cardiac output to increase oxygen delivery, mechanical ventilation may not
only improve PaCO2
but also improve PaO2
by decreasing oxygen consumption. The benefits of supplemental oxygen therapy must
be weighed against the potential adverse physiologic effects of prolonged administration
of a high FIO2
, including denitrogenation
absorption atelectasis and direct cytotoxic effects.[3]
[4]
 |
 Figure 75-1
Schematic representation of the pathogenesis of acute
respiratory failure (ARF). The ventilatory pump is composed of the chest cage, ventilatory
muscles, and nervous system elements involved in respiration. The pump primarily
affects carbon dioxide excretion (CO2
). The lung involves the elements
that allow inspired gas to exchange with pulmonary blood flow and primarily affects
blood oxygenation (O2
). The large arrow from lung to pump represents
the finding that lung disease often increases the work of the pump.
Figure 75-1
Schematic representation of the pathogenesis of acute
respiratory failure (ARF). The ventilatory pump is composed of the chest cage, ventilatory
muscles, and nervous system elements involved in respiration. The pump primarily
affects carbon dioxide excretion (CO2
). The lung involves the elements
that allow inspired gas to exchange with pulmonary blood flow and primarily affects
blood oxygenation (O2
). The large arrow from lung to pump represents
the finding that lung disease often increases the work of the pump.