|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A review of the problems of anesthesia under HBO was published as a report to a committee of the American Society of Anesthesiologists.[192] The report explored various issues, including the potential for nitrous oxide to be used as a sole anesthetic.
Anesthesia may be required as a procedure incidental to the hyperbaric exposure. Ross and coworkers[193] considered the problems associated with anesthesia up to 35 ATA to provide care to injured divers while in a saturation diving system (e.g., in North Sea oil fields). They suggested using intravenous anesthetics for general anesthesia rather than gaseous anesthetics because of the problem of pollution of the chamber environment with the latter. Regional anesthesia was recommended whenever possible. The authors noted that muscle relaxants should be titrated to effect because some degree of pressure reversal at around 10 ATA has been reported.
Since the 1960s, anesthesia has been performed at increased ambient pressure with a variety of anesthetics to facilitate surgery for carotid stenosis, [194] to perform cesarean section for fetal distress in a hypoxic patient,[195] for therapeutic lung lavage in patients with alveolar proteinosis,[48] [49] to perform emergency surgery in a saturation dive,[196] to increase oxygenation in open heart surgery[142] and to enhance the effectiveness of irradiation of carcinoma.[197]
The increased ambient pressure in a hyperbaric chamber allows nitrous oxide to be used at partial pressures exceeding its minimum alveolar concentration. [198] Russell and colleagues[199] observed that it was a less than ideal anesthetic during studies in normal volunteers administered 75% nitrous oxide in O2 for 2 to 4 hours in a hyperbaric chamber at an ambient pressure of 2 ATA. Hornbein and associates performed a similar study at 1.87 ATA (82% N2 O).[200] Although induction of anesthesia by nitrous oxide was rapid (less than 60 seconds) in both studies, it was accompanied by tachypnea, tachycardia, hypertension, diaphoresis, muscle rigidity, catatonic jerking of the extremities, eye opening, and opisthotonos. After 2 to 4 hours of anesthesia, subjects emerged rapidly from the anesthetic; however, most subsequently experienced nausea and vomiting, which was often severe. In a study of two volunteers briefly anesthetized with N2 O at pressures up to 2 ATA, Smith and coworkers[198] observed unexpectedly prolonged recovery in one subject.
A potential problem associated with nitrous oxide anesthesia at high ambient pressure is the possibility that tissues could become supersaturated during decompression and allow bubbles to form (decompression illness). This problem was not observed by Russell and colleagues,[199] who used an empirical staged decompression schedule with a decompression stop for 30 minutes at 1.3 ATA while the patients breathed 100% O2 . Bubble formation can occur without decompression if the patient breathes one gas while surrounded by an atmosphere of another gas that is more soluble. For example, breathing air while in a helium-O2 environment at 5 to 7 ATA can lead to urticaria and vestibular dysfunction[201] as a result of rapid diffusion of helium into tissues, which causes local inert gas pressure to exceed ambient pressure (isobaric gas counterdiffusion). This phenomenon can even occur at normal atmospheric pressure if a person breathes nitrous oxide-O2 while surrounded by helium.[202] Therefore, it is imperative that nitrous oxide never be administered in a helium atmosphere.
 Figure 70-13
Performance of an anesthetic vaporizer system at increased
ambient pressure. A, Flow characteristics of a rotameter
system as shown. At 4 ATA the actual delivered flow is less than 60% of the flow
indicated by the rotameter. B, Fluotec vaporizer
output in partial pressure of halothane as a function of ambient pressure. Only
small increases in delivered partial pressure are evident at 3 ATA, at the 2% and
3% settings. (From Committee on Hyperbaric Oxygenation: Fundamentals of
Hyperbaric Medicine [Publication No. 1298]. Washington, DC, National Academy Press,
1966.)
Figure 70-13
Performance of an anesthetic vaporizer system at increased
ambient pressure. A, Flow characteristics of a rotameter
system as shown. At 4 ATA the actual delivered flow is less than 60% of the flow
indicated by the rotameter. B, Fluotec vaporizer
output in partial pressure of halothane as a function of ambient pressure. Only
small increases in delivered partial pressure are evident at 3 ATA, at the 2% and
3% settings. (From Committee on Hyperbaric Oxygenation: Fundamentals of
Hyperbaric Medicine [Publication No. 1298]. Washington, DC, National Academy Press,
1966.)
If a rapid decompression procedure is implemented while the patient continues to breathe an N2 O-O2 mixture, large amounts of dissolved nitrous oxide will move from tissues and blood into the lungs and possibly cause a significant degree of diffusion hypoxia. Therefore, an O2 -enriched breathing mix should be administered for several minutes before decompression.
Inhaled anesthesia of any type will pollute the enclosed chamber atmosphere with anesthetic gases and may exert pharmacologic effects on medical personnel inside the chamber, particularly at high ambient pressures. Russell and coauthors [199] reported nitrous oxide concentrations of 2500 ppm in chamber air; ventilation of the chamber with air at a high rate (3500 L/min of air) was required to reduce the concentration to 25 to 75 ppm.
In patients who have recently engaged in scuba diving or have suffered decompression illness, nitrous oxide should be avoided, even at 1 ATA, because its administration may result in tissue or blood bubble growth and recrudescence of pain or neurologic symptoms. Such a case in a patient in whom neurologic symptoms developed after a nitrous oxide anesthetic following apparent spontaneous resolution of decompression illness, has been reported by Acott and Gorman.[203]
Halothane has been the most commonly administered volatile anesthetic because it has been found to be both safe and effective in a hyperbaric environment. Although the effect of increased ambient pressure in reversing anesthesia is minimal at 6 ATA, there are effects of increased ambient pressure on the behavior of vaporizer systems.
The effect of a volatile anesthetic on a patient is proportional not to the alveolar concentration but to the partial pressure of the anesthetic. For example, the effect of 1% halothane at 1 ATA (with a partial pressure of 7.6 mm Hg) will be equivalent to a 0.5% concentration at 2 ATA (with the same partial pressure). The anesthetic concentration of an anesthetic-specific calibrated vaporizer varies with ambient pressure, but in a manner such that the partial pressure delivered remains almost constant ( Fig. 70-13 and Fig. 70-14 ).
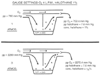 Figure 70-14
Behavior of an ideal Fluotec vaporizer at 1 and 3 ATA.
If the ratio of the O2
flow (anesthetic vaporizer flow/bypass flow) is
unchanged at different ambient pressures, it can be seen that the partial pressure
of halothane delivered at the exit of the vaporizer is constant. (From Smith
RM: Anesthesia during hyperbaric oxygenation. Ann N Y Acad Sci 117:768, 1965.)
Figure 70-14
Behavior of an ideal Fluotec vaporizer at 1 and 3 ATA.
If the ratio of the O2
flow (anesthetic vaporizer flow/bypass flow) is
unchanged at different ambient pressures, it can be seen that the partial pressure
of halothane delivered at the exit of the vaporizer is constant. (From Smith
RM: Anesthesia during hyperbaric oxygenation. Ann N Y Acad Sci 117:768, 1965.)
Rotameter flow meters calibrated at 1 ATA will indicate falsely
high values at increased ambient pressure because of the increased gas density.
McDowell[204]
reported the following relationship
for rotameter flow:
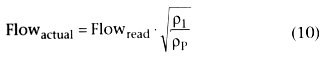
where Flowactual
and Flowread
are the actual and scale reading
flows and ρ1
and ρP
are the gas densities at 1 ATA
and P ATA, respectively. This inaccuracy in rotameter performance up to 4 ATA has
been confirmed by others (see Fig.
70-13
).[192]
|
|
|
|
|
|
|
|
|
|
|
|
|