Mitral Valve Surgery
In 1997, two independent groups reported the first robotically
assisted mitral valve repair.[45]
[46]
In November 2002, the FDA approved the use of robot-assisted surgery in performing
mitral valve repairs. Mitral valves repair, initially done through mini-thoracotomy
incisions, could be done completely with a closed chest. However, mitral valve replacements
may still require a small thoracotomy to introduce the new prosthetic valve.
Anesthetic Implications for Mitral Valve Surgery
Mitral valve surgery employing robotic devices is being done at
a few cardiac centers in the United States and Europe. The anesthetic techniques
and other relevant considerations have been described previously.[47]
Patients are initially evaluated by cardiac catheterization to estimate the degree
of coronary artery stenosis and to assess valve function. Severe mitral regurgitation
is a mechanical problem that requires surgery for cure. Most patients are medically
treated with afterload reducers, such as angiotensin-converting enzyme (ACE) inhibitors
if they are hypertensive. An enlarged left atrium is often susceptible to atrial
fibrillation. Patients with persistent atrial fibrillation may be taking anticoagulants
concomitantly with therapy for rate control. Chronic elevation in left atrial pressure
may manifest with pulmonary hypertension, which may be further exacerbated by obstructive
lung disease. Severe pulmonary hypertension renders a patient unsuitable for robotic
surgery.[48]
Patients are provided with a large peripheral intravenous line.
Light sedation with midazolam and local anesthesia is offered before the placement
of bilateral radial arterial lines. The patient is routinely monitored with ECG
leads II and V5
, pulse oximetry and a right radial artery pressure line
to exclude endovascular aortic balloon misplacement. Modern ECG monitors can provide
automatic ST segment analysis for the detection of ischemia. After ample oxygenation,
the patient is anesthetized with a combination of midazolam, fentanyl, and isoflurane.
On muscle relaxation, the trachea is intubated with a double-lumen endotracheal
tube ( Table 66-1
). Proper
tube position is confirmed by bronchoscopy. A TEE
TABLE 66-1 -- One-lung ventilation strategy
|
Use FIO2
= 1.0. |
|
Begin one-lung ventilation with pressure control ventilation,
maintaining a plateau pressure of <30 cm H2
O. |
|
Adjust the respiratory rate so that PaCO2
approaches 40 mm Hg. |
|
Check arterial blood gas pressure. |
|
Apply continuous positive airway pressure to nonventilated lung. |
|
Apply positive end-expiratory pressure to ventilated lung. |
probe is inserted to assess heart and valve function and to guide central line placement.
A mid-esophageal, bicaval view at 90 degrees is used for guidance in positioning
the superior vena cava (SVC) and inferior vena cava (IVC) cannulas ( Fig.
66-7
). Initially, a left, 9-Fr introducer catheter is inserted by means
of the Seldinger technique, and an 8-Fr pulmonary artery catheter is floated into
the pulmonary artery. Next, the right neck is prepared
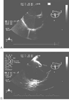 Figure 66-7
A, Ultrasound image of
the superior vena cava cannula. B, Ultrasound image
of a bicaval view depicting the inferior vena cava containing a J guidewire. Both
views are helpful in correctly placing cardiopulmonary bypass venous cannulas.
Figure 66-7
A, Ultrasound image of
the superior vena cava cannula. B, Ultrasound image
of a bicaval view depicting the inferior vena cava containing a J guidewire. Both
views are helpful in correctly placing cardiopulmonary bypass venous cannulas.
for insertion of a percutaneous, 17-Fr Biomedicus cannula. It is inserted directly
into the internal jugular vein using the Seldinger technique, and its proper placement
is confirmed by TEE. Experience shows that the long transthoracic aortic cross-clamp
may impinge and occlude the SVC. For this reason, an armored SVC neck cannula provides
resistance to occlusion or kinking. At the time of insertion, the cannula is flushed
with 5000 units of heparin to ensure its patency. The cannula is anchored with a
purse-string suture at the skin and secured with Kerlix gauze wrapped around the
patient's head.
After the patient's pelvis is positioned supine and the right
shoulder is tilted 30 degrees to the left, transcutaneous defibrillation and pacing
pads are applied. The surgeon can then determine proper location for port access,
which may vary according to a patient's body habitus.
After the right femoral vessels are exposed and left-sided, single-lung
ventilation is established, a right-sided mini-thoracotomy incision is made. The
heart is exposed after a pericardial opening is made. The pericardium is anchored
open to the chest wall by two percutaneous stay sutures. After the patient is heparinized
based on an activated clot time (ACT)-guided protocol, the femoral vein and artery
are cannulated in anticipation of femoral-femoral cardiopulmonary bypass. First,
the femoral vein is cannulated, and a 21-Fr cannula is placed over a guidewire and
passed into the IVC-RA junction with the aid of TEE. One end hole and 12 side holes
resist collapse under the high negative pressure that is created by augmented venous
return pumps. Likewise, the femoral artery is cannulated with a 24-Fr cannula, and
cardiopulmonary bypass is initiated with venous drainage from the femoral and jugular
veins. Anterograde and retrograde cardioplegia cannulas are placed. Some surgical
teams prefer to cannulate the ascending aorta using a Heartport Straight-shot.[48]
A transthoracic aortic cross-clamp is passed percutaneously through the right axilla
and applied to the ascending aorta. The robotic arms are engaged through their respective
trocars lateral to the mini-thoracotomy incision while the camera arm passes directly
through the thoracotomy incision. The left atrium can be entered for mitral valve
repair or replacement.
Before terminating cardiopulmonary bypass, TEE is used to evaluate
the function of the mitral valve, residual valvular regurgitation and to confirm
the disappearance of intracardiac air. The anterior leaflet of the mitral valve
is further inspected for systolic anterior motion.
Patient selection is important for optimal results. Table
66-2
lists the risk factors that make patients unsuitable candidates for
robotic mitral valve surgery.
 Figure 66-7
A, Ultrasound image of
the superior vena cava cannula. B, Ultrasound image
of a bicaval view depicting the inferior vena cava containing a J guidewire. Both
views are helpful in correctly placing cardiopulmonary bypass venous cannulas.
Figure 66-7
A, Ultrasound image of
the superior vena cava cannula. B, Ultrasound image
of a bicaval view depicting the inferior vena cava containing a J guidewire. Both
views are helpful in correctly placing cardiopulmonary bypass venous cannulas.