Early Resuscitation
Fluid administration is the cornerstone of acute resuscitation.
Intravascular volume is lost because of hemorrhage,
uptake by ischemic cells, and extravasation into the interstitial space. Administration
of intravenous fluids will predictably increase cardiac output and BP in a hypovolemic
trauma patient. The ATLS curriculum advocates the rapid infusion of up to 2 L of
warmed isotonic crystalloid solution for any hypotensive patient, with the goal of
restoring normal BP and urine output.[62]
This approach has been questioned in recent years because of a
growing awareness that fluid administration to a patient who is actively hemorrhaging
may be counterproductive. Dilution of red cell mass reduces oxygen delivery and
contributes to both hypothermia and coagulopathy. Elevation of BP leads to increased
bleeding as a result of disruption of clots and reversal of compensatory vasoconstriction.
[63]
The result of aggressive fluid administration
is often a transient rise in BP, followed by increased bleeding and another episode
of hypotension, followed by the need for more volume administration. This vicious
cycle has been recognized since the First World War and remains a complication of
resuscitation therapy today. Resuscitation must therefore be considered in two phases:
- • Early, while the patient is still actively
hemorrhaging, and
- • Late, once all hemorrhage has been controlled
Managing late resuscitation is driven by end point targets and
consists of giving enough fluid to maximize the patient's oxygen delivery. Early
resuscitation is much more complex because the risks of aggressive volume replacement
summarized in Table 63-6
,
including the potential for exacerbating hemorrhage and thus prolonging the crisis,
must be weighed against the risk of ongoing hypoperfusion.
Deliberate hypotensive management is an accepted standard of anesthetic
care for elective surgical cases such as total-joint replacement, spinal fusion,
radical neck dissection, reconstructive facial surgery, and major pelvic or abdominal
procedures.[64]
Application of this technique to
the initial management of a hemorrhaging trauma victim is highly controversial and
has been the focus of numerous laboratory and clinical research efforts. In 1965
Shaftan published the results of a study of native coagulation mechanisms and demonstrated
that formation of a soft extraluminal clot limits bleeding after arterial trauma.
[65]
This study compared the quantity of blood
lost
from a standardized arterial injury in dogs under a variety of
*Most
complications of volume resuscitation arise from increased hemorrhage volume or excessive
hemodilution.
conditions, including maintenance of BP with vasoconstrictors, hypovolemia induced
by prebleeding, vasodilatation with a chemical agent, and replacement of lost blood
with immediate transfusion. The least blood loss occurred in hypotensive animals
(whether hypotensive from hemorrhage or from vasodilator administration), followed
by the control group, followed by vasoconstricted animals. The largest amount of
blood was lost in animals that received vigorous reinfusion during the period of
hemorrhage.
A large body of laboratory data have shown the benefits of limiting
fluid administration to actively hemorrhaging animals.[66]
[67]
[68]
[69]
In the most sophisticated models, direct assessment of cardiac output and regional
perfusion showed no difference between moderate- or large-volume resuscitation in
cardiac output, BP, or regional perfusion in the heart, kidneys, and intestines.
Moderate resuscitation (to a lower than normal BP) improved perfusion of the liver.
[70]
Burris and coworkers studied both conventional
resuscitation fluids and various combinations of hypertonic saline and dextran and
found that rebleeding was correlated with higher mean arterial pressure (MAP) and
that survival was best in groups resuscitated to a lower than normal MAP. The optimal
target BP for resuscitation varied with the composition of the fluid used.[71]
A 1994 consensus panel on resuscitation from hemorrhagic shock noted that mammalian
species are capable of sustaining MAP as low as 40 mm Hg for periods as long as 2
hours without deleterious effects. The panel concluded that spontaneous hemostasis
and long-term survival were maximized by reduced administration of resuscitation
fluids during the period of active bleeding while seeking to keep perfusion only
above the threshold for ischemia.[72]
Two prospective studies of deliberate hypotensive resuscitation
in trauma patients have been published. The first was that of Bickell and colleagues
in 1994.[73]
[74]
The investigators randomized victims of penetrating torso trauma to one of two treatment
groups: standard of care (up to 2L of crystalloid infused in the prehospital setting)
or delayed resuscitation (no fluid until reaching the OR). This well-controlled
37-month study eventually included 598 patients. Average times of transport and
care were 30 minutes from injury to the ED and then 50 minutes before reaching the
OR; the fluid-restricted group received an average of about 800 mL of fluid in this
time. The immediate-resuscitation group received an average of 2500 mL of crystalloid
and 130 mL of blood over this same period. Though substantially different during
the period of study, BP on arrival at the OR was similar in both groups, which the
authors took as evidence that the unresuscitated group had achieved spontaneous hemostasis.
The unresuscitated group went on to receive less fluid intraoperatively than the
immediate-resuscitation group did, but this difference was not statistically significant.
Survival to hospital discharge in the delayed-resuscitation group was significantly
improved over the immediate-resuscitation group (70% versus 62% [P
= .04]). No data were presented on the conduct of anesthesia after arrival at the
OR but before control of hemorrhage or on the incidence of rebleeding after volume
loading and induction of anesthesia in patients who had achieved hemostasis preoperatively.
A retrospective review of trauma admissions to Los Angeles Medical
Center published in 1996 supported these findings. Patients brought to the hospital
by private conveyance, without prehospital resuscitation, fared substantially better
than those delivered by paramedics, even at high levels of injury severity.[75]
Further corroboration was provided by retrospective examination of outcomes in a
population of hemorrhaging trauma patients who received fluids by means of a commercial
rapid infusion system (RIS, Haemonetics, Inc.) during initial resuscitation.[76]
The survival rate of this group was compared with the survival predicted by the
institution's trauma registry, with results as presented in Table
63-7
. RIS patients, when subsequently compared with case-matched controls,
had a survival rate of only 56.8% versus 71.2% in patients of similar age, with similar
injuries (P < .001).
This retrospective review was followed in 2002 by the second prospective
trial of delayed resuscitation in trauma patients.[77]
Patients with systolic BP less than 90 mm Hg and clinical evidence of blood loss
were randomized to fluid resuscitation titrated to a systolic BP of 100 mm Hg (normal
group) or 70 mm Hg (study group) until the end of surgical interventions to control
hemorrhage. The results of this study are summarized in Table
63-8
. As in the Bickell study, hypotension allowed for spontaneous resolution
of hemorrhage and autoresuscitation; BP would rise without exogenous fluid administration
once hemostasis was achieved. The typical patient began with a low initial pressure,
followed by recovery to the vicinity of the target, overshoots and undershoots as
bleeding and fluid administration continued, and an eventual rise above the target
when the hemorrhage resolved, even in the absence of further fluid administration
( Fig. 63-8
). The 93% overall
survival rate in this study was higher than predicted from historical data and substantially
higher than that seen in Bickell's group. This higher rate reflects the exclusion
of patients who died in the prehospital phase or who arrived at the trauma resuscitation
unit in a moribund condition. It may also reflect improvements in overall care,
an observation effect (i.e., patients in both groups received better care than did
patients not in the
*Calculation
of the probability of survival was based on published historical methodology.
study), or a bias in subject recruitment. Although deliberate hypotensive resuscitation
did not improve mortality, there was evidence that the patients in the low-pressure
group were more severely injured, with a greater depth of shock at the time of initial
evaluation, than the patients in the normal-pressure group. This difference, which
occurred through random chance, introduced a significant bias in the study results.
Over the first 24 hours, the lactate and base deficit cleared to normal in both
groups, with similar amounts of fluid and blood products required, thus suggesting
that both groups were reaching an equivalent resuscitation end point. The low-pressure
group had a significantly longer stay in intensive care and in the hospital. This
result was driven by a small number of patients in the low-pressure group who had
MOSF. It was unclear whether
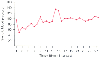 Figure 63-8
Typical systolic blood pressure measurements of a patient
undergoing damage control surgery for a grade V liver injury during deliberate hypotensive
management. Oscillations in blood pressure are common during early resuscitation
because of ongoing hemorrhage and bolus fluid administration. Once hemorrhage is
controlled, blood pressure will stabilize.
Figure 63-8
Typical systolic blood pressure measurements of a patient
undergoing damage control surgery for a grade V liver injury during deliberate hypotensive
management. Oscillations in blood pressure are common during early resuscitation
because of ongoing hemorrhage and bolus fluid administration. Once hemorrhage is
controlled, blood pressure will stabilize.
the MOSF was due to the deliberate delay in resuscitation or to survival in patients
who would otherwise have exsanguinated. The authors concluded that administration
of fluids to an actively hemorrhaging patient should be titrated to specific physiologic
end points, with the anesthesiologist navigating a course between the Scylla of increased
hemorrhage and the Charybdis of hypoperfusion.
The effect of anesthetics on the body's response to hemorrhage
is an important difference between deliberate hypotension occurring in the elective
operative setting and hemorrhagic shock occurring in the ED. Hypotensive humans
commonly receive a minimum of sedating or analgesic agents, even for induction, because
of the obvious effect of these drugs on BP. A hypotensive trauma patient is thus
in a state of profound vasoconstriction, as opposed to a patient undergoing elective
intraoperative hypotension, who is deliberately vasodilated. Table
63-9
summarizes the physiologic contrasts between these two states. Blood
loss without shock does not produce systemic complications such as ARDS in experimental
models.[56]
Based on this physiology, the recommended
goals for early resuscitation are expressed in Table
63-10
, and an algorithm for management is provided in Figure
63-9
. The emphasis in this situation must be on rapid diagnosis and control
of ongoing hemorrhage; the anesthesiologist should attempt to restore vascular volume
and provide anesthesia in equal measure such that the patient is moved from a vasoconstricted
state to a vasodilated one while facilitating hemostasis by maintenance of a lower
than normal BP.
 |
 Figure 63-8
Typical systolic blood pressure measurements of a patient
undergoing damage control surgery for a grade V liver injury during deliberate hypotensive
management. Oscillations in blood pressure are common during early resuscitation
because of ongoing hemorrhage and bolus fluid administration. Once hemorrhage is
controlled, blood pressure will stabilize.
Figure 63-8
Typical systolic blood pressure measurements of a patient
undergoing damage control surgery for a grade V liver injury during deliberate hypotensive
management. Oscillations in blood pressure are common during early resuscitation
because of ongoing hemorrhage and bolus fluid administration. Once hemorrhage is
controlled, blood pressure will stabilize.