|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Resuscitation is an active process that commences immediately after injury, beginning with the patient's own compensatory mechanisms, and continues for hours or even days thereafter. Resuscitation, in a broad sense, refers to the restoration of normal physiology after injury. Resuscitation from hemorrhagic shock refers specifically to the restoration of normal circulating blood volume, normal vascular tone, and normal tissue perfusion.
The initial response to traumatic hemorrhage is on the macrocirculatory level and is mediated by the neuroendocrine system. Decreased BP leads to vasoconstriction and catecholamine release. Heart, kidney, and brain blood flow is preserved, whereas other regional beds are constricted. Pain, hemorrhage, and cortical perception of traumatic injuries lead to the release of a number of hormones, including renin-angiotensin, vasopressin, antidiuretic hormone, growth hormone, glucagon, cortisol, epinephrine, and norepinephrine.[41] This response sets the stage for the microcirculatory responses that follow.
Individual ischemic cells respond to hemorrhage by taking up interstitial fluid, thereby further depleting intravascular fluid.[42] Such uptake may choke off adjacent capillaries and result in a "no-reflow" phenomenon that prevents the reversal of ischemia even in the presence of adequate macroperfusion. [43] Ischemic cells produce lactate and free radicals, which accumulate in the circulation if perfusion is diminished. These compounds cause direct damage to the cell, as well as form the bulk of the toxic load that will be washed back to the central circulation when flow is reestablished. The ischemic cell will also produce and release a variety of inflammatory factors: prostacyclin, thromboxane, prostaglandins, leukotrienes, endothelin, complement, interleukins, tumor necrosis factor, and others.[44] Figure 63-7 shows the inflammatory response to shock and demonstrates the amplification that occurs once the immune system is triggered. It is worth noting that the inflammatory response, once begun, becomes a disease process independent of its origin. This is why a patient may die of multiple organ failure after traumatic hemorrhage, even when bleeding has been controlled and the patient resuscitated to normal vital signs and perfusion.
Specific organ systems respond to traumatic shock in specific ways. The CNS is the prime trigger of the neuroendocrine response to shock, which maintains perfusion to the heart, kidney, and brain at the expense of other tissues.[45] Regional glucose uptake in the brain changes during shock.[46] Reflex activity and cortical electrical activity are both depressed during hypotension; these changes are reversible with mild hypoperfusion but become permanent with prolonged ischemia. Failure to recover preinjury neurologic function is a marker for a poor prognosis, even when normal vital signs are restored.[47]
The kidney and adrenal glands are prime responders to the neuroendocrine changes associated with shock; these organs produce renin, angiotensin, aldosterone, cortisol, erythropoietin, and catecholamines.[48] The kidney itself maintains glomerular filtration in the face of hypotension by selective vasoconstriction and concentration of blood flow in the medulla and deep cortical area. Prolonged hypotension leads to decreased cellular energy
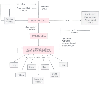 Figure 63-7
The "shock cascade." Ischemia in any given region of
the body will trigger an inflammatory response that will have an impact on nonischemic
organs even after adequate systemic perfusion has been restored. (Redrawn
from Dutton RP: Shock and trauma anesthesia. In
Grande CM, Smith CE [eds]: Anesthesiol Clin North Am: Trauma 17:83–95. Philadelphia:
WB Saunders, 1999.)
Figure 63-7
The "shock cascade." Ischemia in any given region of
the body will trigger an inflammatory response that will have an impact on nonischemic
organs even after adequate systemic perfusion has been restored. (Redrawn
from Dutton RP: Shock and trauma anesthesia. In
Grande CM, Smith CE [eds]: Anesthesiol Clin North Am: Trauma 17:83–95. Philadelphia:
WB Saunders, 1999.)
The heart is relatively preserved from ischemia during shock because of maintenance or even an increase in nutrient blood flow, and cardiac function is generally well preserved until the late stages. Lactate, free radicals, and other humoral factors released by ischemic cells all act as negative inotropes and, in a decompensated patient, may produce cardiac dysfunction as the terminal event in the shock spiral.[50] A patient with cardiac disease or direct cardiac trauma is at great risk for decompensation because a fixed stroke volume inhibits the body's ability to increase blood flow in response to hypovolemia and anemia. Tachycardia is the patient's only option, with potentially disastrous consequences for the oxygen supply-demand balance in the heart itself. Shock in the elderly may therefore be rapidly progressive and may not respond predictably to fluid administration.[51]
The lung is the destination for the inflammatory byproducts of an ischemic body. Accumulation of immune complex and cellular factors in pulmonary capillaries leads to neutrophil and platelet aggregation, increased capillary permeability, destruction of lung architecture, and the acute respiratory distress syndrome (ARDS).[52] [53] The lung is typically the sentinel organ for the development of multiple organ system failure (MOSF) in traumatic shock patients.[54] [55] Pure hemorrhage, in the absence of hypoperfusion, does not produce pulmonary dysfunction.[56] This is evidence that traumatic shock is more than just a hemodynamic disorder.
The gut is one of the earliest organs affected by hypoperfusion and may be the prime trigger of MOSF. Intense vasoconstriction occurs early and frequently leads to a "no-reflow" phenomenon even when the macrocirculation is restored.[57] Intestinal cell death causes a breakdown in the barrier function of the gut that results in increased translocation of bacteria to the liver and lung, thereby potentiating ARDS.[58] The liver has a complex microcirculation and has been demonstrated to suffer reperfusion injury during recovery from shock.[59] Hepatic cells are also metabolically active and contribute to the ischemic inflammatory response and to irregularities in blood glucose.[60] Failure of the synthetic functions of the liver after shock are almost always lethal. Skeletal muscle is not metabolically active during shock and tolerates ischemia better than other organs do. The large mass of skeletal muscle, though, makes it important in the generation of lactate and free radicals from ischemic cells. Sustained ischemia of muscle cells leads to an increase in intracellular sodium and free water with an aggravated depletion of fluid in the vascular and interstitial compartments.[61]
|
|
|
|
|
|
|
|
|
|
|
|
|