|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Isotonic crystalloids (normal saline, lactated Ringer's solution [LR], Plasma-Lyte A) are the initial resuscitative fluids administered to any trauma patient. They have the advantage of being inexpensive, readily available, nonallergenic, noninfectious, and efficacious in restoring total-body fluid. They are relatively easy to store and administer, they mix well with most infused medications, and they can be rapidly warmed to body temperature. Disadvantages of crystalloids include their lack of oxygen-carrying capacity, their lack of coagulation capability, and their limited intravascular half-life. More recent laboratory data have implicated specific crystalloid solutions as immunosuppressants and triggers of cellular apoptosis.[82] Unlike necrosis, apoptosis is highly regulated and involves gene modulation and complex pathways of signal transduction. It seems clear that apoptosis is an important element of reperfusion injury. In a rat model of controlled hemorrhage, animals receiving LR solution showed an immediate increase in apoptosis in the liver and small intestine after resuscitation with LR. [83] Neither whole blood nor hypertonic saline increased the amount of apoptosis.
Hypertonic saline solutions, with or without the addition of polymerized dextran (HS or HSD), have been extensively studied in resuscitation from hemorrhagic shock.[84]
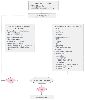 Figure 63-9
Algorithm for the management of early hemorrhagic shock.
ABCs, airway, breathing, circulation; ABG, arterial blood gases; CBC, complete blood
count; HCT, hematocrit; PT, prothrombin time; SBP, systolic blood pressure.
Figure 63-9
Algorithm for the management of early hemorrhagic shock.
ABCs, airway, breathing, circulation; ABG, arterial blood gases; CBC, complete blood
count; HCT, hematocrit; PT, prothrombin time; SBP, systolic blood pressure.
Colloids, including hetastarch solutions and albumin, have long been advocated for rapid plasma volume expansion. Like crystalloids, colloids are readily available, easily stored and administered, and relatively inexpensive. As with the hypertonic solutions, colloids will increase intravascular volume by drawing free water back into the vascular space. When intravenous access is limited, colloidal resuscitation will restore intravascular volume more rapidly than crystalloid infusion will and at a lower volume of administered fluid. Because colloids do not specifically transport oxygen or facilitate clotting, their dilutional effect on blood will be similar to that of crystalloids. Studies have demonstrated no great benefit of colloids over crystalloids in a variety of resuscitation models.[86]
Many of the risks of aggressive fluid administration just summarized are related to dilution of circulating blood volume. Recognition of this fact and continued improvement in the safety of donated blood have led to increased use of blood products in the management of early hemorrhagic shock (also see Chapter 47 ). The risk of systemic ischemia is decreased by the maintenance of an adequate hematocrit, and the potential for dilutional coagulopathy can be avoided with the early administration of plasma. The composition of resuscitation fluids may be as important as the rate and timing of administration. A 4-year retrospective review of a cohort of critically injured patients who underwent emergency surgery examined the outcomes of short-term care based on the number of units of blood transfused.[87] One hundred forty-one patients received massive blood transfusions (20 U or more of packed red blood cells [PRBCs]) during preoperative and intraoperative resuscitation. The number of blood units did not differ between survivors (30%) and nonsurvivors (70%). Eleven variables were significantly different: aortic clamping for control of BP, use of inotropic drugs, time with systolic BP less than 90 mm Hg, time in the OR, temperature lower than 34°C, urine output, pH less than 7.0, PaO2 /FIO2 ratio less than 150, PaCO2 higher than 50 mm Hg, potassium greater than 6 mM/L, and calcium less than 2 mM/L. Of these variables, the presence of the first three in the face of transfusion of more than 30 U of PRBCs was invariably fatal. Total blood loss and the amount of transfused blood were less critical than the depth and duration of shock. These concerns led to the concept of damage control surgery, which emphasizes rapid control of active hemorrhage.[88]
PRBCs are the mainstay of treatment of hemorrhagic shock. With an average hematocrit of 60% to 70%, a unit of PRBCs will predictably restore oxygen-carrying capacity and expand intravascular volume as well as any colloid solution will. PRBCs of blood type A, B, or AB carry major incompatibility antigens that may precipitate a lethal transfusion reaction if given to a patient with the wrong blood type. Because PRBCs also carry dozens of minor antigens that can cause reactions in susceptible patients, crossmatching is desirable when time allows (typically about 1 hour from the time that a sample reaches the blood bank until the PRBCs reach the patient). Type-specific blood requires less time to deliver from the blood bank (usually about 30 minutes) and may be an appropriate alternative in some situations. Type O blood—the "universal donor"—can be given to patients of any blood type with little risk of a major reaction. This approach is preferred in patients who arrive at the ED already in hemorrhagic shock; major trauma centers will maintain a supply of type O blood immediately available for transfusion in this population. O-negative blood will not sensitize women of childbearing age to the rhesus antigen, but it is less common than O-positive blood and therefore harder to maintain in inventory. If O-positive blood is given in this situation, prophylactic administration of anti-Rn0 antibody is indicated.
Risks of PRBC administration include transfusion reaction, transmission of infectious agents, and hypothermia. PRBCs are stored at 4°C and will lower the patient's temperature rapidly if not infused through a warming device or mixed with warmed isotonic crystalloid at the time of administration. Premixing with crystalloid will also reduce the viscosity of PRBCs and allow more rapid administration. Viral infection transmission rates have been significantly reduced in recent years, with current estimates of risk being 1:63,000 for any agent, 1:150,000 for hepatitis C, and 1:1,000,000 for human immunodeficiency virus.[89] Crossmatched blood carries a risk for major transfusion reaction of less than 1:100,000 (largely because of clerical error); the risk for type-specific or type O blood is quoted at 1:100 to 1:10,000, although no hemolytic transfusion reactions were observed in more than 100,000 transfusions of uncrossmatched blood during the Vietnam War. [90] Repeat crossmatching at the conclusion of resuscitation is recommended for patients who have received massive transfusions.
Administration of plasma is indicated for the treatment of coagulopathy that arises during resuscitation from hemorrhagic shock. Like PRBCs, plasma is an excellent volume expander. Plasma carries a risk for transmission of infectious diseases similar to that of PRBCs and must also be warmed during administration, especially early in resuscitation. Plasma is not usually necessary with transfusion requirements of 1 to 4 U of PRBCs; most patients will have sufficient coagulation factor reserves to compensate for this amount of blood loss. Patients who reach the massive transfusion threshold (one blood volume or about 10 U of PRBCs) will generally require 1 U of plasma for each unit of PRBCs. The need for plasma when between 5 and 9 U of PRBCs is transfused is variable. Coagulation parameters (prothrombin time or international normalized ratio, partial thromboplastin time, fibrinogen) should be measured frequently during resuscitation. Plasma should be administered to correct any deviation from normal in an actively hemorrhaging patient because this condition can spiral downward very quickly: hemorrhage exacerbates coagulopathy, thereby begetting further hemorrhage. Plasma and PRBCs should be administered prophylactically in a 1:1 ratio to any patient with obvious massive hemorrhage, even before confirmatory laboratory studies are available. Plasma requires blood typing but not crossmatching; delay in the availability of plasma is caused by the need to thaw frozen units before they can be administered. Busy university and urban hospitals will often maintain a supply of prethawed plasma (thawed fresh plasma as opposed to fresh frozen plasma) that can be issued quickly in response to an emergency need; in other hospitals, it is important to request plasma early in resuscitation if it is likely to be needed. Cryoprecipitate or specific coagulation factor preparations are not indicated for the treatment of dilutional coagulopathy in the absence of a known congenital factor deficiency.
Platelet transfusion should be reserved for clinically coagulopathic patients with a documented low serum level (<50,000 cells/mm3 per high-power field). Although the development of coagulopathy in elective surgical patients is usually the result of platelet deficiency, the same is not true in trauma patients, who are more apt to suffer from consumption of coagulation factors. Transfused platelets have a very short serum half-life and should generally be administered only to patients with visible coagulopathy. Platelets should not be administered
Rapid transfusion of banked blood carries the risk of inducing "citrate intoxication" in the recipient.[91] Every component unit is packaged with one of several anticoagulation agents (citrate being a common choice) that bind free calcium, an essential requirement of the clotting cascade. Consecutive administration of multiple units of banked blood overwhelms the body's ability to mobilize free calcium and causes a marked reduction in circulating serum calcium with a profound negative inotropic effect on the heart. Unrecognized hypocalcemia is a common cause of hypotension that persists despite an adequate volume of resuscitation. Ionized calcium levels should be measured at regular intervals in a hemorrhaging patient, and calcium should be administered as needed (in a separate intravenous line from that for transfusion products) to maintain serum levels in the normal range.
Hemoglobin-based oxygen carriers (HBOCs) (also see Chapter 47 ) have been proposed as an alternative to PRBCs in acute resuscitation from hemorrhagic shock. Advantages of HBOCs include longer shelf-life, lower cost, no need for crossmatching, and minimal risk of viral transmission. Concerns with HBOCs include the potential for inappropriate vasoconstriction and hypertension as a result of increased scavenging of nitric oxide and potentiation of coagulopathy because of platelet impairment. The only human trial in acute trauma patients did not substantiate these side effects, but it also failed to demonstrate a survival benefit with the use of HBOCs.[92] At smaller doses, HBOCs may facilitate delivery of oxygen to ischemic tissue. Trials examining this indication in prehospital resuscitation are under way at this time.
|
|
|
|
|
|
|
|
|
|
|
|
|