|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The approach to intravenous fluid therapy in a child must consider
the high metabolic demands and the high ratio of body surface area to weight. The
basis for calculating
| Weight (kg) | Hourly Fluid Requirements (mL) | 24-hr Fluid Requirements (mL) |
|---|---|---|
| <10 | 4 mL/kg | 100 mL/kg |
| 11–20 | 40 mL + 2 mL/kg > 10 kg | 1000 mL + 50 mL/kg > 10 kg |
| >20 | 60 mL + 1 mL/kg > 20 kg | 1500 mL + 20 mL/kg > 20 kg |
The composition of the intravenous fluid is also a concern. Because greater hypoxic brain damage was found to occur in animals with high blood glucose levels, some anesthesiologists recommend not using glucose-containing solutions routinely, especially for brief operative procedures.[274] However, a concern about unrecognized hypoglycemia was the motivating factor regarding the routine use of
Despite limited data, modification of current practice can be proposed for long surgical procedures or for patients thought to be at risk for hypoglycemia. A balanced salt solution (e.g., lactated Ringer's solution) should be used for all deficits and third-space losses and 5% dextrose in 0.45% normal saline administered by "piggyback" infusion at maintenance rates to minimize the chance of a bolus administration of glucose and satisfy the concern for unrecognized hypoglycemia or accidental hyperglycemia. The routine use of 5% dextrose and lactated Ringer's solution as the initial replacement for fluid deficit and for maintenance is discouraged. For most patients, lactated Ringer's solution is the only fluid required.
Children receiving intravenous alimentation present a special problem. Transition to 10% glucose is often recommended to avoid intraoperative hyperglycemia with continuation of the current alimentation fluid and to avoid hypoglycemia if it is stopped suddenly. However, this change to 10% glucose has not been adequately studied. It would seem reasonable to reduce the established intravenous alimentation fluid infusion rate by 40% to 50% and to periodically check blood glucose values. This then does not waste the current alimentation fluid and involves only a simple change in the infusion rate.
Fluid management of term neonates and premature infants must take into account other variables. The amount of insensible water loss is inversely proportional to gestational age. The younger and more physically immature the patient, the higher the skin permeability, the ratio of body surface area to weight, and the metabolic demand. In addition, the use of radiant warmers and phototherapy increases insensible water loss. On the other hand, the use of a heated humidifier and warm air mattress preserves body heat and reduces insensible water loss.
The fact that the neonatal kidney is unable to excrete large amounts of excess water or electrolytes must also be considered. As described earlier, the volume of extracellular fluid in a newborn is very large. During the first days of life, some of this excess water is excreted. Therefore, term newborns have reduced fluid requirements for the first week of life. The daily fluid requirements for a term newborn in the days after birth are as follows: day 1, 70 mL/kg; day 3, 80 mL/kg; day 5, 90 mL/kg; and day 7, 120 mL/kg. Daily fluid requirements would be slightly higher for a premature infant. Sodium and potassium concentrations are usually kept at 2 to 3 mEq/100 mL.
Newborns are usually started on 10% glucose to prevent hypoglycemia. This fluid is continued for the first several days of life until glucose values are stable. Infants of diabetic mothers or mothers given large amounts of glucose just before delivery may require greater concentrations of glucose for protection against rebound hypoglycemia. Infants unable to tolerate oral feeding may continue to receive intravenous 10% glucose solutions and may even require peripheral or central alimentation. A number of infants have been transferred to our institution with severe and potentially life-threatening hyperglycemia (i.e., blood glucose levels of 700 to 900 mg/dL) as a result of overzealous use of this type of glucose therapy. This solution should be used with caution if infants require surgery, and it is unclear that 10% glucose in water is still necessary at that time. Every effort should be made to reduce bolus administration of glucose (an infusion pump should be used); blood sugar levels should be monitored and the solution infused at maintenance rates.
The use of blood products in pediatric surgical patients has diminished greatly because of the fear of transmission of disease—particularly human immunodeficiency virus (HIV). Because HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and a number of other disease-causing viruses can be transmitted with as little as 10 mL of packed red blood cells (PRBCs), administration of any blood product requires clear, medically defensible clinical indications that preferably are recorded on the anesthetic record. The current risk for transmission of HIV, HBV, or HCV is estimated to be 1 in 50,000 to 1,900,000 U (see www.aabb.org/All_About_Blood/FAQs/aabb_faqs.htm). [278] [279] Because of these concerns, it is common for families to donate blood for their child or relative; in this situation, the blood product should be irradiated to prevent possible graft-versus-host disease.[280] [281]
When caring for children, it is very important to think in terms
of blood volumes and portions of blood volumes lost rather than units because a unit
of blood may constitute several blood volumes in a premature infant but only a fraction
of the blood volume of a robust teenager. These considerations govern calculation
of the maximal allowable blood loss (MABL) resulting in an acceptable hematocrit.
The MABL takes into account the effect of patient age, weight, and starting hematocrit
on blood volume. In general, blood volume is approximately 100 to 120 mL/kg for
a premature infant, 90 mL/kg for a full-term infant, 80 mL/kg for a child 3 to 12
months old, and 70 mL/kg for a child older than 1 year. These are merely estimates
of blood volume. The individual patient's blood volume is calculated by simple proportion
by multiplying the patient's weight by the estimated blood volume (EBV) per kilogram.
Although several formulas are available, a simple relationship is easiest to remember:
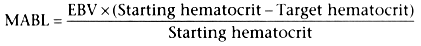
Thus, if a 3-year-old child weighs 15 kg and has a starting hematocrit of 38% and
if clinical judgment estimates the desired postoperative hematocrit to be 25%, the
calculation would be as follows:
MABL = (15 × 70) × (38 − 25)/38 =
1050 × 13/38 ![]() 360 mL
360 mL
MABL would then be replaced with 3 mL of lactated Ringer's solution per milliliter
of blood loss, that is, 3 mL
If the child has reached the MABL and significantly more blood
loss is expected during surgery, the child should receive PRBCs in sufficient quantity
to maintain the hematocrit in the 20% range. The entire loss of red cell mass should
not be replaced (unless clinically indicated) if these additional PRBCs would expose
the child to additional units of blood products. Hematocrit values in the low 20%
range are usually well tolerated by most children, the exception being premature
infants, term newborns, and patients with cyanotic congenital heart disease or those
with respiratory failure in need of a high oxygen-carrying capacity. Because the
incidence of apnea is higher in neonates and premature infants who have hematocrit
values below 30%, it would be prudent to discuss with both the surgeon and neonatologist
the minimal target hematocrit for such patients and to document the discussion in
the patient record.[282]
[283]
Older children with a history of sickle cell disease may require preoperative transfusion
and should be managed in conjunction with their attending hematologist.[284]
[285]
A simple formula for intraoperative or postoperative
estimation of the volume of PRBCs needed in the 15-kg child in the earlier example
with a hematocrit of 20% is

Fresh frozen plasma (FFP) is administered to replenish clotting factors lost during massive blood transfusion (usually defined as exceeding one blood volume), for disseminated intravascular coagulopathy, or for congenital clotting factor deficit. The anesthesiologist initiates and guides therapy with FFP during massive blood loss, whereas the hematologist's advice is sought when either of the other two conditions exists.
Patients with known clotting factor deficits, such as those with massive thermal injury or coagulopathy, may require transfusion of FFP before blood loss exceeds one blood volume. In contrast, healthy patients who do not have coagulation factor deficits at the beginning of surgery do not need FFP until blood loss exceeds 1 and probably 1.5 blood volumes.[286] This generalization applies to patients given PRBCs; patients given whole blood will not need FFP, even when blood loss exceeds several blood volumes.[287] Despite a blood loss of one blood volume, prolongation of the prothrombin time (PT) and the partial thromboplastin time (PTT) will only be minor.[286] [288]
Blood loss exceeding 1 to 1.5 blood volumes (replaced entirely with PRBCs and crystalloid, albumin, or other nonblood products) often necessitates transfusion of FFP. However, the decision to administer FFP should be based on observed coagulopathy and documented prolongation of the PT and PTT. Obtaining these test results from the laboratory often takes longer than desired. In this circumstance, a note should be written that blood loss has exceeded one blood volume and that abnormal oozing has occurred in the surgical field. A patient should never be given FFP to correct bleeding that is surgical in nature.
No studies on children have yet clearly defined what values for PT and PTT are associated with pathologic bleeding sufficient to require transfusion of FFP to replace clotting factors. However, if associated with abnormal oozing, a PT exceeding 15 seconds (international normalized ratio [INR] > 1.4) or a PTT longer than 60 seconds (>1.5 baseline) seems to warrant correction.[289] If these abnormalities exist but oozing does not occur and the surgical field is relatively safe from the complications of hematoma formation (e.g., as in orthopedic surgery versus neurosurgery), it seems appropriate to observe the patient and withhold transfusion of FFP.
The volume of FFP required to correct prolongation of the PT and PTT depends on the severity of the clotting factor deficit and the presence or absence of consumptive coagulopathy. In general, FFP therapy may require replacement of 30% or more of the patient's blood volume. Transfusion of FFP at rates exceeding 1.0 mL/kg/min is sometimes followed by severe ionized hypocalcemia and cardiac depression with hypotension, especially if FFP is administered during anesthesia with a potent inhaled anesthetic ( Fig. 60-13 ). [290] [291] Therefore, exogenous calcium chloride (2.5 to 5 mg/kg) or calcium gluconate (7.5 to 15 mg/kg) should be administered during rapid transfusion of FFP.[292] Ionized hypocalcemia occurs very frequently in neonates given FFP, possibly because of their decreased ability to mobilize calcium and metabolize citrate; patients undergoing liver transplantation or those with compromised hepatic function or perfusion may also be at increased risk because of a decreased ability to metabolize citrate.[293]
Thrombocytopenia may occur as a consequence of disease processes (idiopathic thrombocytopenic purpura, chemotherapy, infection, disseminated intravascular coagulopathy) or as a result of dilution during massive blood loss. Children whose platelet count has fallen because of idiopathic thrombocytopenic purpura or chemotherapy generally tolerate platelet counts as low as 15,000/mm3 without a need for platelet transfusion. In contrast, patients whose platelet count has decreased because of dilution (massive blood loss) generally require platelet transfusion when the count is 50,000/mm3 or less.[294] The reason for this disparity is not known. However, in my experience, the preoperative platelet count has been extremely valuable
 Figure 60-13
Ionized hypocalcemia always accompanies the administration
of citrated blood products (fresh frozen plasma, citrated whole blood). Fresh frozen
plasma has the highest concentration of citrate per unit volume of any blood product
and is the most likely to cause ionized hypocalcemia during rapid infusion. Studies
in children with severe thermal injuries suggest that rates exceeding 1.0 mL/kg/min
produce severe ionized hypocalcemia. If no additional citrated blood products are
administered, this abnormality corrects itself because of metabolism of the citrate.
However, patients with impaired hepatic blood flow (infants, liver transplant patients,
trauma patients) may need exogenous calcium therapy. *P
< .001; †P < .0021 versus baseline.
(From Coté CJ, Drop LJ, Hoaglin DC, et al: Ionized hypocalcemia
after fresh frozen plasma administration to thermally injured children: Effects
of infusion rate, duration, and treatment with calcium chloride. Anesth Analg 67:152–160,
1988.)
Figure 60-13
Ionized hypocalcemia always accompanies the administration
of citrated blood products (fresh frozen plasma, citrated whole blood). Fresh frozen
plasma has the highest concentration of citrate per unit volume of any blood product
and is the most likely to cause ionized hypocalcemia during rapid infusion. Studies
in children with severe thermal injuries suggest that rates exceeding 1.0 mL/kg/min
produce severe ionized hypocalcemia. If no additional citrated blood products are
administered, this abnormality corrects itself because of metabolism of the citrate.
However, patients with impaired hepatic blood flow (infants, liver transplant patients,
trauma patients) may need exogenous calcium therapy. *P
< .001; †P < .0021 versus baseline.
(From Coté CJ, Drop LJ, Hoaglin DC, et al: Ionized hypocalcemia
after fresh frozen plasma administration to thermally injured children: Effects
of infusion rate, duration, and treatment with calcium chloride. Anesth Analg 67:152–160,
1988.)
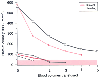 Figure 60-14
Dilutional thrombocytopenia occurs whenever massive blood
loss takes place. The need for platelet transfusion, however, depends on the starting
platelet count. In patients who started with low platelet counts, dilutional thrombocytopenia
developed after blood loss of one or two blood volumes, whereas patients who started
with very high platelet counts did not require transfusions of platelets. The bottom
three sets of points represent patients with initial low platelet counts, and the
top two sets of points represent children with initial
high platelet counts. (Data from Coté CJ, Liu LM, Szyfelbein SK,
et al: Changes in serial platelet counts following massive blood transfusion in
pediatric patients. Anesthesiology 62:197–201, 1985.)
Figure 60-14
Dilutional thrombocytopenia occurs whenever massive blood
loss takes place. The need for platelet transfusion, however, depends on the starting
platelet count. In patients who started with low platelet counts, dilutional thrombocytopenia
developed after blood loss of one or two blood volumes, whereas patients who started
with very high platelet counts did not require transfusions of platelets. The bottom
three sets of points represent patients with initial low platelet counts, and the
top two sets of points represent children with initial
high platelet counts. (Data from Coté CJ, Liu LM, Szyfelbein SK,
et al: Changes in serial platelet counts following massive blood transfusion in
pediatric patients. Anesthesiology 62:197–201, 1985.)
Whenever platelets are administered, the anesthesia record should document the reason for the transfusion, and every effort should be made to obtain a platelet count before transfusion. Clinical oozing is the typical indication for platelet transfusion, unless the potential for bleeding would be critical to survival, such as during neurosurgery, cardiac surgery, or major organ transplantation. The initial volume of platelets transfused is approximately 0.1 to 0.3 U/kg; the increase in platelet count obtained with this amount of transfusion varies considerably and depends on the presence or absence of platelet antibodies and the rate of platelet consumption.
A fluid/blood warmer is essential for any patient who might require rapid correction of intravascular volume. The use of such devices for maintenance intravenous fluid therapy does not provide any benefit because the rate of infusion is so slow that the intravenous fluid returns to room temperature between the time that it exits the warmer and enters the patient. New warming devices that use countercurrent or microwave warming methods are superior to the older water bath coiled tubing devices. Low-capacity passive flow through devices such as the Hot Line (Level 1 Technologies, Inc., Rockland, MA) are useful, but this device can allow air embolization.[295] [296] High-capacity active flow systems such as the Level 1 System 1000 (Level 1 Technologies) and the Rapid Infusion System (RIS, Haemonetics Corporation, Braintree, MA) are capable of delivering large volumes of blood or crystalloid in a brief period.[297] [298] The latter device can deliver up to 1500 mL/min.[299] Our experience with these devices is that there is no difference between the Level 1 System 1000 and the RIS device in warming or flow capacity for crystalloids (we did not test colloids) with intravenous catheters 18 gauge or smaller, but for every catheter larger than 18 gauge, the RIS was superior in terms of warming and volume delivered. However, for most pediatric cases, the Level 1 System 1000 is more than adequate because the size of the intravenous catheter and its length limit
|
|
|
|
|
|
|
|
|
|
|
|
|