DEVELOPMENTAL PHYSIOLOGY OF THE INFANT
Organogenesis takes place within 8 weeks of conception, organ
function develops during the second trimester, and the infant gains weight, primarily
muscle and fat, during the third trimester.[1]
Any physiologic or pharmacologic injury or stress during the first trimester may
cause abnormal organogenesis; in the second trimester, it may cause abnormal functional
development of organs and, in the third trimester, smaller organs or reduced muscle
and fat mass.[2]
Injuries and stress take the form
of congenital viral infections, exposure to drugs, nutritional insufficiency (caloric
or vascular), or other maternal illness. A genetic predisposition to developmental
malformations can also produce adverse effects. Such interruptions in normal growth
and development may cause a variety of physiologic abnormalities ranging from simple
premature birth to a constellation of congenital malformations.[3]
[4]
[5]
[6]
A premature infant is one born before 37 weeks of gestation; a
postmature infant is one born after 42 weeks of gestation. Any infant less than
2500 g is considered a low-birth-weight infant. Plotting weight against gestational
age allows classification into three general categories: small for gestational age,
appropriate for gestational age, or large for gestational age ( Fig.
60-1
). Infants who are small or large for gestational age often have developmental
problems or difficulties associated with maternal disease ( Table
60-1
). A careful physical and neurologic examination at birth allows a
fairly accurate estimate of gestational age. The anesthesiologist should be aware
of this type of evaluation so that potential problems can be anticipated. A perinatal
history regarding problems during pregnancy (e.g., maternal drug abuse, maternal
infection, eclampsia, diabetes) or during and after delivery (e.g., fetal distress,
meconium aspiration, postdelivery intubation) is also valuable for assessing possible
anesthetic complications and specific considerations for anesthetic management.
In the weeks after birth, measures of weight, height, and head circumference are
plotted on standard developmental curves; deviations from the normal
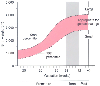 Figure 60-1
Plotting birth weight against gestational age for neonates
determines whether infants are small, appropriate, or large for gestational age.
Babies who are either small or large for gestational age are particularly likely
to have a variety of problems such as metabolic, developmental, infectious, or structural
abnormalities, as well as drug addiction and withdrawal. (Redrawn with modification
from Battaglia FC: Intrauterine growth retardation. Am J Obstet Gynecol 106:1103–1114,
1970.)
Figure 60-1
Plotting birth weight against gestational age for neonates
determines whether infants are small, appropriate, or large for gestational age.
Babies who are either small or large for gestational age are particularly likely
to have a variety of problems such as metabolic, developmental, infectious, or structural
abnormalities, as well as drug addiction and withdrawal. (Redrawn with modification
from Battaglia FC: Intrauterine growth retardation. Am J Obstet Gynecol 106:1103–1114,
1970.)
(i.e., crossing developmental lines) usually indicate a severe physiologic insult.
The anesthesiologist should examine the growth chart to evaluate how the child is
developing.
The Cardiovascular System
The cardiovascular system undergoes dramatic physiologic and maturational
changes during the first year of life. In utero, most of the cardiac output is directed
from the placenta across the foramen ovale into the ascending aorta (oxygenated blood),
whereas superior vena cava blood (deoxygenated) is directed to both the pulmonary
artery and the ductus arteriosus. This pattern of circulation results in minimal
intrauterine pulmonary blood flow. At birth, a number of events change hemodynamic
interactions such that the fetal circulation becomes an adult-type circulation.[7]
Specifically, the placenta is removed from the circulation; portal blood pressure
falls, which causes the ductus venosus to close and blood becomes oxygenated through
the lungs; and exposure of the ductus arteriosus to oxygenated blood induces ductal
closure. As a result of the combined effects of lung expansion, exposure of blood
to oxygen, and loss of low resistance through placental blood flow, pulmonary vascular
resistance decreases while peripheral vascular resistance rises rapidly. The fall
in pulmonary vascular resistance occurs on the first day of life and continues to
decrease gradually during the next several years as the architecture of the pulmonary
vessels changes. An increase in pressure on the left side of the heart (caused by
the rise in peripheral vascular resistance) induces mechanical closure of the foramen
ovale. Thus all three connections between the right and left side
TABLE 60-1 -- Common neonatal problems associated with weight and gestational age
|
Gestational Age |
Body Size |
Increased Incidence of These Neonatal Problems |
|
Premature |
Small |
Respiratory distress syndrome |
|
|
Apnea |
|
|
Hypoglycemia |
|
|
Hypomagnesemia |
|
|
Hypocalcemia |
|
|
Fetal alcohol syndrome |
|
|
Viral infection |
|
|
Thrombocytopenia |
|
|
Congenital anomalies |
|
|
Maternal drug addiction |
|
|
Neonatal asphyxia |
|
|
Aspiration pneumonia |
|
Term |
Small |
Congenital anomalies |
|
|
Viral infection |
|
|
Thrombocytopenia |
|
|
Maternal drug addiction |
|
|
Neonatal asphyxia |
|
|
Hypoglycemia |
|
|
Fetal alcohol syndrome |
|
Postmature |
Small |
Congenital anomalies |
|
|
Viral infection |
|
|
Thrombocytopenia |
|
|
Maternal drug addiction |
|
|
Neonatal asphyxia |
|
|
Aspiration pneumonia |
|
|
Hypoglycemia |
|
|
Fetal alcohol syndrome |
|
Any gestational age |
Large |
Birth trauma |
|
|
Hyperbilirubinemia |
|
|
Hypoglycemia: infant of diabetic mother |
|
|
Transposition of great arteries |
|
Modified from Todres ID: Growth and development.
In Coté CJ, Ryan JF, Todres ID, et al (eds):
A Practice of Anesthesia for Infants and Children, 2nd ed. Philadelphia, WB Saunders,
1993, p 76. |
of the circulation close. Although closure of the ductus arteriosus probably occurs
primarily in response to a rise in arterial oxygen concentration, its successful
completion requires arterial muscular tissue.[8]
The fact that such tissue is less prevalent in premature infants may account, in
part, for the high incidence of patent ductus arteriosus in premature infants. True
mechanical closure by fibrosis does not occur until 2 to 3 weeks of age.[7]
[8]
During this critical period, the infant readily reverts from the
adult circulation to a fetal type of circulation; this state is called transitional
circulation. Many factors (hypoxia, hypercarbia, anesthesia-induced changes in peripheral
vascular tone) can affect this precarious balance and result in a sudden return to
fetal circulation. When such a "flip-flop" occurs, pulmonary artery pressure increases
to systemic levels, blood is shunted past the lungs through the patent foramen ovale,
and the ductus arteriosus may
reopen and allow blood to shunt at the ductal level. A rapid downhill spiral may
occur and result in severe hypoxemia; this explains why hypoxemic events in infants
are often prolonged despite adequate pulmonary ventilation with 100% oxygen.
Risk factors increasing the likelihood of a prolonged transitional
circulation include prematurity, infection, acidosis, pulmonary disease resulting
in hypercarbia or hypoxemia (aspiration of meconium), acidosis, hypothermia, and
congenital heart disease. Care must be directed to keeping the infant warm, maintaining
normal arterial oxygen and carbon dioxide tension, and minimizing anesthetic-induced
myocardial depression.
The myocardial structure of the heart, particularly the volume
of cellular mass devoted to contractility, is significantly less developed in neonates
than adults. These differences, as well as developmental changes in contractile
proteins, produce a leftward displacement of the cardiac function curve and less
compliant ventricles. This developmental myocardial immaturity accounts for the
tendency toward biventricular failure, sensitivity to volume loading, poor tolerance
of increased afterload, and heart rate-dependent cardiac output.[9]
[10]
Another issue is that cardiac calcium stores
are reduced because of immaturity of the sarcoplasmic reticulum; consequently, the
neonate has a greater dependence on exogenous calcium and probably increased susceptibility
to myocardial depression by potent inhaled drugs that have calcium channel blocking
activity.[11]
[12]
 Figure 60-1
Plotting birth weight against gestational age for neonates
determines whether infants are small, appropriate, or large for gestational age.
Babies who are either small or large for gestational age are particularly likely
to have a variety of problems such as metabolic, developmental, infectious, or structural
abnormalities, as well as drug addiction and withdrawal. (Redrawn with modification
from Battaglia FC: Intrauterine growth retardation. Am J Obstet Gynecol 106:1103–1114,
1970.)
Figure 60-1
Plotting birth weight against gestational age for neonates
determines whether infants are small, appropriate, or large for gestational age.
Babies who are either small or large for gestational age are particularly likely
to have a variety of problems such as metabolic, developmental, infectious, or structural
abnormalities, as well as drug addiction and withdrawal. (Redrawn with modification
from Battaglia FC: Intrauterine growth retardation. Am J Obstet Gynecol 106:1103–1114,
1970.)