|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pertinent details of the surgical procedure are also discussed in the section "Intraoperative Management." Lung transplantation may be considered to begin with a pneumonectomy performed through either a standard posterolateral thoracotomy incision or a bilateral thoracosternotomy incision. With the assistance of one-lung ventilation, the lung is dissected and the hilar structures identified, clamped, and divided. After the native lung is removed, the lung graft is then situated within the hemithorax, and anastomosis of the hilar structures is begun. Typically, hilar attachment begins with the bronchial anastomosis, followed by the arterial anastomosis. A clamp placed on the recipient's left atrium facilitates attachment of the venous cuff, frequently the most technically challenging anastomosis. Deviation from this order is common and of minor importance to the anesthesiologist. Reperfusion occurs with removal of the arterial clamp. De-airing maneuvers are often performed at this time, followed by removal of the left atrial clamp. With resumption of two-lung ventilation and assurance of adequate hemostasis, the procedure is repeated on the contralateral side (BSLT) or else concluded with closure of the wound (SLT) ( Fig. 56-6A–B and Fig. 56-6C–D ).
Patients who present for lung transplantation will frequently have been waiting in excess of 2 years and will have undergone a thorough assessment and follow-up examination by surgical and pulmonary specialists. As with any unscheduled surgical procedure, the anesthesiologist must also evaluate the patient for potential problems that may be encountered with anesthesia care.
Except with HLT, or concomitant repair of a cardiac defect, the vast majority of lung transplants are performed with CPB as a discretionary option. Although many transplant teams avoid CPB whenever possible in light of both real and theoretical risks to the patient and graft, proponents of the routine use of CPB during lung transplantation recognize facilitated airway management (lung separation may not be necessary), improved surgical exposure, and intraoperative respiratory and hemodynamic stability as compelling advantages. Elective use of CPB is nearly always undertaken for patients with severe pulmonary hypertension—those with a mean PAP greater than 40 mm Hg.[368] [375] [376] [377] [378] [379]
Patients with very poor RV systolic function may not tolerate unilateral pulmonary artery cross-clamping[378] and may therefore undergo CPB proactively. The preoperative evaluation of RV function and PAP (obtained by cardiac catheterization or echocardiography) should be reviewed, and a plan regarding the use of CPB should be developed in consultation with the surgeon.
Although most isolated lung transplantation procedures can be managed without CPB, it is always possible that extracorporeal support will be needed on an emergency basis. For this reason, the procedure is best performed with "CPB standby," or readily available CPB equipment and personnel. Patients with COPD who present for SLT infrequently require unplanned CPB,[368] [378] whereas those with IPF undergoing the same surgery may demonstrate the need in 17% to 41% of cases.[378] [380] During BSLT, 26% to 32% of patients may require unplanned CPB.[377] [378]
Because the use of CPB is known to be associated with increased blood loss, blood product use, and re-thoracotomy,[338] [379] [381] [382] [383] administration of antifibrinolytic
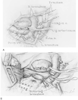 Figure 56-6a
A, The left hilum is shown.
The pulmonary artery and veins are divided, and the intact mainstem bronchus lies
posteriorly. Division of the bronchus will allow completion of the recipient pneumonectomy.
B, The donor lung is positioned within the recipient's
left hemithorax. In this case, the hilar anastomosis begins with the left mainstem
bronchus. (From Shumway SJ, Shumway NE: Operative techniques in lung transplants.
In Thoracic Transplantation. Oxford, Blackwell
Scientific, 1995, pp 183–186.)
Figure 56-6a
A, The left hilum is shown.
The pulmonary artery and veins are divided, and the intact mainstem bronchus lies
posteriorly. Division of the bronchus will allow completion of the recipient pneumonectomy.
B, The donor lung is positioned within the recipient's
left hemithorax. In this case, the hilar anastomosis begins with the left mainstem
bronchus. (From Shumway SJ, Shumway NE: Operative techniques in lung transplants.
In Thoracic Transplantation. Oxford, Blackwell
Scientific, 1995, pp 183–186.)
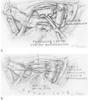 Figure 56-6b
C, The left hilar anastomosis
is nearly complete. A left atrial clamp permits anastomosis of the pulmonary veins
with an appropriately sized cuff of the left atrium. D,
On completion of the hilar anastomosis, the lung is reperfused and de-airing maneuvers
are performed. Ventilation is initiated, and the procedure is repeated contralaterally
in the case of bilateral transplants.
Figure 56-6b
C, The left hilar anastomosis
is nearly complete. A left atrial clamp permits anastomosis of the pulmonary veins
with an appropriately sized cuff of the left atrium. D,
On completion of the hilar anastomosis, the lung is reperfused and de-airing maneuvers
are performed. Ventilation is initiated, and the procedure is repeated contralaterally
in the case of bilateral transplants.
The fact that a delivery system for inhaled NO is now commercially available has provided the opportunity for it to be used intraoperatively during lung transplantation. NO is discussed further in the next section.
The timing of the induction of anesthesia is usually based on close communication with the surgeon or the transplant coordinator. The interval between donor lung explantation and completion of the hilar anastomoses within the recipient (donor lung ischemic time) may affect postoperative pulmonary function. [374] Adherence to proper aseptic technique throughout the perioperative period is advocated because of the added susceptibility to infection while receiving immunosuppressive therapy. Infection is the second most frequent cause of early postoperative death in this population.[285] Before induction of anesthesia, cannulation of the femoral vessels should be considered in preparation for the emergency institution of CPB. Such preparation may be important in patients with significant pulmonary hypertension and severe RV dysfunction, who are, accordingly, at increased risk for hemodynamic collapse with the onset of anesthesia and positive-pressure ventilation.[385]
If postoperative neuraxial analgesia is planned, the best time for injection of subarachnoid opioids or placement of an epidural catheter is probably before induction of anesthesia,[386] although some have preferred to postpone this until the postoperative period. Neuraxial analgesia for lung transplantation is discussed further in the next section.
In addition to excellent intravenous access, including at least two large-bore intravenous catheters and standard monitors, appropriate adjuncts include arterial, central, and pulmonary arterial pressure monitoring. Placement of a pulmonary artery catheter provides the means to measure cardiac output, which when combined with PAP monitoring, may aid in the detection and assessment of hemodynamic compromise at critical times during the operation, such as clamping of the hilar pulmonary artery. The decision whether to place invasive
Because the transplant procedure is usually unscheduled, patients may be incompletely fasted. Rapid-sequence induction or "awake" intubation should be considered. A slower, controlled induction may be safely performed in those requiring utmost stability at induction by administering an oral antacid (such as sodium citrate) and using cricoid pressure throughout. The choice of anesthetics is relatively unimportant when compared with the requirement for maintenance of ventricular contractility and avoidance of increased PVR. Drugs associated with significant myocardial depression, such as propofol or halothane, are best avoided in those with severe RV dysfunction. Factors that are known to aggravate pulmonary hypertension, such as hypoventilation (with associated hypercapnia and acidemia), hypoxemia, stimulation during light anesthesia, and hypothermia, should also be avoided. The slow titration of opioids (such as fentanyl or sufentanil) in combination with other drugs that lack significant negative inotropic effect (such as etomidate) may provide hemodynamic stability in those judged to be at high risk. Although volatile anesthetics are generally well tolerated, especially when combined with minimum alveolar concentration (MAC)-lowering intravenous drugs, nitrous oxide is disfavored because of its tendency to increase PVR, aggravate the significance of intravascular air, and substantially limit FIO2 when administered at clinically relevant doses. While avoiding hypoventilation (i.e., during manual ventilation after induction), care should be taken to avoid excessive airway pressure, especially in those with severe pulmonary hypertension or bullous parenchymal disease. Endotracheal intubation is facilitated by using a neuromuscular blocking drug, the choice of which is based on standard considerations, in addition to avoiding histamine-releasing drugs (e.g., atracurium) because of their tendency to precipitate bronchoconstriction. Patients undergoing HLT will benefit from similar principles of induction. Please see the section "Cardiac Transplantation" for an expanded discussion of patients with end-stage cardiac disease.
Consideration should be given to temporary placement of a standard single-lumen endotracheal tube to facilitate fiberoptic bronchoscopic assessment of the tracheobronchial tree and allow thorough suctioning in patients with CF. SLT or BSLT, however, will require single-lung ventilation. Although any accepted technique can be used, the double-lumen endobronchial tube, which has the ability to selectively ventilate either lung, is a good choice. Left-sided endobronchial tubes may generally be used for BSLT or SLT, regardless of the planned side of engraftment.
Patients are positioned supine for HLT and BSLT procedures and in the lateral position for SLT. Typically, the surgical approach for HLT is through a midline sternotomy, whereas BSLT is most often performed through a bilateral thoracosternotomy (clamshell) incision. SLT is generally performed through a posterolateral thoracotomy incision similar to that used for the common pneumonectomy procedure.
After induction and endotracheal intubation, the initial ventilatory strategy should take into account the patient's predominant ventilatory pathophysiology, which may be either obstructive or restrictive in nature. It is important to remember that those with PPH or Eisenmenger's syndrome may have fairly normal pulmonary mechanics. Those with obstructive ventilatory defects will benefit from lower respiratory rates and lower inspiratory-to-expiratory (I:E) ratios, both of which maximize expiratory time. "Breath stacking" may result from a sustained pattern of tidal volume delivery with an insufficient expiratory time that leads to incomplete exhalation of the preceding tidal volume. This phenomenon may result in increased airway pressure, hypoventilation, or barotrauma and may be a cause of hypotension in patients with an obstructive physiology, particularly those with significant RV dysfunction.[388] [389] Hypotension occurs in part as a result of impaired caval blood flow in the face of increased intrathoracic pressure, but also because of overdistension of pulmonary capillary beds causing high RV afterload. Many COPD patients have a preexisting baseline elevation in PaCO2 and an FEV1 of less than 25% of the predicted value.[368] To pursue normocapnia during one-lung ventilation often results in unacceptable airway pressure, breath stacking, or both. Consequently, many anesthesiologists adopt a strategy of permissive hypercapnia in which a safe balance is struck between ventilatory mechanics and CO2 elimination.[388] [389] [390] The safe limit of hypercapnia during lung transplantation is not known; levels of PaCO2 as high as 80 to 90 mm Hg may be well tolerated,[388] [391] and levels of up to 162 mm Hg have been reported [389] without postoperative sequelae. Acidemia, myocardial depression, and ventricular dysrhythmia can occur in association with hypercapnia, which may affect the degree of hypercapnia that is tolerated.
Patients with predominantly restrictive physiology, such as those with IPF, are not generally subject to gas trapping or breath stacking, but instead have decreased parenchymal compliance, which leads to relatively high airway pressure under anesthesia with positive-pressure ventilation. The generation of tidal volumes that are otherwise considered appropriate may require unacceptably high peak inspiratory airway pressure. The ventilatory strategy may include a significant decrease in tidal volume along with a commensurate increase in rate. Limited use of positive end-expiratory pressure (PEEP) (i.e., 4 to 8 cm H2 O) may help prevent the loss of recruited alveoli (atelectasis), whereas a higher I:E ratio (i.e., 1:1.5) or even
For SLT or BSLT procedures that are performed without elective
CPB, surgical and anesthetic management begins as for pneumonectomy. Single-lung
ventilation (to the nonoperative lung) is initiated at or soon after pleural entry
to facilitate dissection of the lung that is to be removed. When ventilator settings
cannot sustain acceptable oxygenation maneuvers to improve V̇/![]() matching,
options may include the application of continuous positive airway pressure (e.g.,
5 to 10 cm H2
O) to the nonventilated lung or similar quantities of PEEP
to the ventilated lung. In patients with copious airway secretions, such as those
with CF, bronchial suctioning should be repeated and can be facilitated by fiberoptic
bronchoscopy. In refractory situations, clamping or ligation of the nonventilated
lung's pulmonary artery may improve oxygenation. If clamping or ligation becomes
necessary, the pulmonary artery catheter should first be withdrawn from the branch
pulmonary artery.
matching,
options may include the application of continuous positive airway pressure (e.g.,
5 to 10 cm H2
O) to the nonventilated lung or similar quantities of PEEP
to the ventilated lung. In patients with copious airway secretions, such as those
with CF, bronchial suctioning should be repeated and can be facilitated by fiberoptic
bronchoscopy. In refractory situations, clamping or ligation of the nonventilated
lung's pulmonary artery may improve oxygenation. If clamping or ligation becomes
necessary, the pulmonary artery catheter should first be withdrawn from the branch
pulmonary artery.
Unplanned CPB may be needed at any time, such as in response to surgical mishaps[375] [377] or to complete a difficult anastomosis satisfactorily[379] [381] (completion of the left atrial-to-pulmonary venous cuff anastomosis during left lung transplantation is frequently the most challenging). However, CPB is most characteristically prompted by deterioration of gas exchange during one-lung ventilation or by hemodynamic decompensation at the time of pulmonary artery clamping. The deterioration in gas exchange may be gradual, or ventilation failure may occur rapidly (e.g., with development of a bronchopleural fistula at the site of a ruptured bulla). Hemodynamic deterioration necessitating the need for CPB may be manifested as severe RV dysfunction with decreased cardiac output or systemic hypotension. Pulmonary hypertension per se may or may not precipitate RV failure and low cardiac output, but it may become very severe (i.e., with pressures greater than two thirds of systemic blood pressure) with pulmonary artery clamping. This possibility alone should prompt one to consider using CPB. Increased RV pressure may limit coronary perfusion while at the same time increase oxygen consumption, and systolic function may not be sustainable for the period of implantation.[381] Some authors have recommended that CPB be instituted should the cardiac index fall by 1.5 L/min/m2 or more after placement of the pulmonary artery clamp. [380] Pharmacologic support may include inotropic drugs to improve RV contractility and pulmonary vasodilators to reduce PVR. The use of common intravenous pulmonary vasodilators such as nitroglycerin may be limited by systemic vasodilation and hypotension or by inhibition of hypoxic pulmonary vasoconstriction, which can potentially aggravate hypoxemia.
In contrast, inhaled NO may improve RVR, RV function, and oxygenation during lung transplantation without lowering systemic vascular resistance or blood pressure.[392] [393] Of major importance, however, is the question of whether inhaled NO may improve hemodynamics and gas exchange to the extent of avoiding CPB in situations in which it would otherwise have been necessary. Although such occurrences have been reported, [394] little is otherwise known regarding such potential.
After removal of the native lung, the transplant typically proceeds with the bronchial anastomosis, followed by the arterial and venous connections. Generally, donor pulmonary veins are excised with a cuff of donor left atrium that is attached to the recipient's left atrium. (Lobes donated from living donors do not include atrial cuffs, although the veins are left as long as possible to facilitate anastomosis with the recipient's pulmonary vein.) Intermittent reinflation of the graft may assist with de-airing of the pulmonary veins before removal of the left atrial clamp. If available, TEE will help identify any significant air within the left atrium, ventricle, and outflow tract after reestablishment of pulmonary blood flow.
Allograft reperfusion begins at the time of unclamping of the pulmonary artery. Typical immunosuppression regimens call for bolus administration of intravenous corticosteroid immediately before reperfusion. There is usually a reduction in mean PAP and PVR and, with the concomitant resumption of bilateral lung ventilation, improvement in gas exchange and overall pulmonary mechanics.[393] If a second transplant is planned, dissection of the remaining native lung begins, and this procedure is facilitated by single-lung ventilation of the newly implanted donor lung.
Hypotension at the time of reperfusion may result from relative hypovolemia as the graft is perfused or from systemic washout of prostaglandin and preservative solution. Of greater concern, however, is that newly transplanted lungs may exhibit features of the pulmonary reimplantation response (PRR), which is characterized by low-pressure pulmonary edema and accompanied by deterioration in compliance, oxygenation, and PVR.
PRR occurs in 15% to 35% of lung transplant recipients and is attributed largely to ischemia-reperfusion injury, possibly aggravated by denervation and loss of lymphatic drainage.[374] It is recognized as the predominant mechanism underlying the clinical syndrome of early (primary) graft dysfunction, which connotes severity sufficient to require prolonged mechanical ventilation.[395] Histologically, there is diffuse alveolar damage with infiltration of polymorphonuclear neutrophils. PRR can occur within minutes to several days after donor lung reperfusion. An intraoperative manifestation of PRR may necessitate unplanned CPB for completion of the second pulmonary allograft. [377] Early graft dysfunction or PRR varies in severity and at times necessitates an increased duration of intensive care and postoperative ventilation[395] while also contributing substantially to the early mortality of lung transplantation.[285]
It has been recognized that reperfusion injury of the first donor lung may be aggravated by pulmonary hypertension and overcirculation during engraftment of the second lung because the first lung must accept the entire cardiac output during the period of contralateral pulmonary artery clamping. This has prompted some to routinely opt for elective CPB during BSLT,[381] [396] often for the second implantation alone.[379] Although such a strategy allows for controlled (i.e., low flow) reperfusion of both the first and second lung, others have suggested that CPB adds to the inflammatory environment within the donor lung, which can potentially exacerbate PRR.[389] [397] [398] [399] Investigators differ on the notion that CPB confers
Interestingly, inhaled NO has been recognized as a drug that may prevent or interrupt the pathophysiologic processes involved in the development of PRR[400] [401] [402] [403] [404] [405] ; however, the clinical studies involving humans have been conflicting. A number of investigators have shown inhaled NO to be efficacious in the management of PRR, both in the operating room and afterward, as evidenced by decreased intrapulmonary shunting, improved arterial oxygenation, and lowered PVR and PAP.[392] [393] [400] [404] [406]
After implantation of the graft or grafts, ventilation is reestablished to both lungs. FIO2 should be minimized to avoid pulmonary oxygen toxicity while at the same time maintaining acceptable systemic oxygenation. The use of PEEP (e.g., 5 cm H2 O) and inhaled NO may facilitate this goal. Coagulopathy (particularly after periods of CPB) may require treatment with platelet, fresh frozen plasma, and red blood cell transfusions to stabilize nonsurgical blood loss and maintain acceptable oxygen-carrying capacity. Pulmonary mechanics may resemble that of normal lungs or may reflect decreased compliance in patients with PRR. Other causes of problems with gas exchange at this stage include bronchial anastomotic stenosis or dehiscence, obstruction at the pulmonary venoatrial connection, and pulmonary edema. Bronchoscopy is useful for evaluation of anastomotic integrity, whereas TEE may reveal pulmonary venous obstruction.[360]
With proper ABO blood group compatibility between the donor and recipient, hyperacute rejection is rare but can occur as a result of preformed alloantibodies within the recipient. These alloantibodies form in response to previous blood transfusions, transplantation, or pregnancy. To minimize the possibility of hyperacute rejection, recipients are prescreened, and those with significant alloantibody reactivity are excluded. Acute rejection is not often manifested during the intraoperative period.
Before leaving the operating room, the endobronchial tube (if used) is removed and replaced with a standard single-lumen tube. The single-lumen tube is necessary to facilitate bronchoscopic inspection of the anastomoses, allow tracheobronchial suctioning, and assist in postoperative ventilatory care in general.
Mechanical ventilation is typically provided for 1 or more days in the absence of complications,[366] [367] [379] but in the presence of early graft dysfunction, it may be prolonged by several weeks.[367] [370] [395] Management consists of supportive mechanical ventilation[406] in which oxygenation is achieved in a manner that minimizes ventilator-induced lung injury. The application of PEEP (5 to 10 cm H2 O) may facilitate this goal while permitting limitation of FIO2 and its potential for pulmonary toxicity. Peak inspiratory pressure should be maintained below 40 cm H2 O whenever possible. Conservative fluid administration is advocated even in the absence of increased left atrial pressure to minimize pulmonary edema in the face of leaky pulmonary capillaries.[370] Central and pulmonary artery wedge pressure should be maintained within the lower ranges consistent with satisfactory cardiac output. Loop diuretics and inotropic infusions are frequently administered to facilitate clearance of excess lung water. Early graft dysfunction may persist after an earlier manifestation of PRR in the operating room, or it may develop in the ICU. Although most cases of PRR are transient (i.e., lack persisting significance), early graft dysfunction probably represents a subset of PRR in which prolonged ventilatory support is required and survival and long-term functional status may be significantly compromised.[395] Inhaled NO has been used successfully in the postoperative management of early graft dysfunction insofar as oxygenation and PVR are improved.[400] [401] [405] [406] In cases of refractory oxygenation failure, ECMO has been used pending improvement in graft function or retransplantation.
Gas exchange failure and hemodynamic compromise have occasionally been caused by dynamic hyperinflation of the retained native lung. Such hyperinflation has been observed in patients with emphysema who have undergone SLT and who experience air trapping in the non-transplanted lung to the extent that the mediastinum and donor lung become compressed. Management of these cases can include double-lumen endobronchial intubation with differential lung ventilation (i.e., a separate ventilator for each lung). This allows for the use of ventilator settings in the native lung that minimize breath stacking and peak airway pressure, such as low tidal volume (2 to 5 mL/kg) and frequencies as low as 4 breaths/min.[395] [407]
Other important causes of respiratory failure in the postoperative setting include acute rejection episodes, bronchial anastomotic stricture or failure, pneumonia, and complications related to bleeding (e.g., cardiac tamponade, hemomediastinum, or hemothorax). Bronchoscopic lavage and biopsy are frequently required to distinguish between infectious and immunologic graft dysfunction because they have similar clinical and radiologic features.
Pain control after lung transplantation is an essential component of anesthetic management, particularly when surgical access has been achieved by thoracotomy or bilateral thoracosternotomy incisions. Intravenous opioid analgesia may provide adequate efficacy and may be preferred in patients in whom neuraxial analgesia is contraindicated, refused, impossible, or ineffective. Neuraxial analgesia for lung transplantation has been achieved with intrathecal and epidural techniques, with the latter having been reported to facilitate earlier tracheal extubation and discharge from the ICU.[408] [409] Epidural neuraxial analgesia may provide better pain relief than achieved with intravenous opioids.[408] Despite concern regarding its safety, it has not been associated with epidural hematoma formation in patients undergoing lung transplantation.[386] [410] Lumbar epidural catheter placement may be appropriate for the administration of hydrophilic, diffusible opioids such as morphine, whereas thoracic-level placement (e.g., T4-7) may deliver mixtures of local anesthetics and opioids (e.g., bupivacaine with fentanyl) closer to the origin of surgical discomfort. Epidural catheter placement may be safest if performed at least 1 hour before
The conduct of anesthesia in patients who have undergone lung transplantation will be influenced by the degree of dysfunction exhibited by the transplanted lung or lungs, as well as the remaining native lung in the case of SLT. Although double-lung transplant patients may enjoy normal or near-normal spirometric function and gas exchange by 9 to 12 months post-transplant, those with a remaining diseased lung will probably have evidence of persisting obstructive or restrictive ventilatory defects, as well as some degree of abnormal oxygenation. Furthermore, allograft function may be compromised at any time by episodes of acute rejection and, with an increasing interval from the time of implantation, by the cumulative effects of chronic rejection (bronchiolitis obliterans). Finally, an elevated risk for acute pulmonary infection accompanies the permanent requirement for immunosuppressive therapy. Accordingly, those presenting for surgery should undergo preoperative assessment of pulmonary status, including chest roentgenography, spirometry, and arterial blood gas measurement. These tests are important to serve not only as a baseline for stable patients but also—in conjunction with suggestive symptoms or functional decline—as a basis for suspicion of intercurrent infection or acute rejection. Fever, malaise, dyspnea, cough, hypoxemia, leukocytosis, and radiographic infiltrates may accompany either entity.[365] [414] Because they are often difficult to distinguish clinically, referral and further investigation (e.g., transbronchial biopsy) may be required to direct management. Regardless, all but the most urgent procedures should be delayed when evidence of such reversible complications is present.
Certain variances from normal pulmonary physiology will be present even in those with well-functioning grafts. Whereas arterial hypercapnia may persist for the first month after transplantation, normalization of arterial CO2 and the ventilatory response to changes in CO2 is usual beyond this time. [365] [414] [415] [416] [417] Oxygenation is likely to be nearly normal in the absence of significant acute or chronic rejection or a significantly diseased contralateral native lung. The loss of afferent and efferent innervation distal to the bronchial anastomosis results, respectively, in loss of the cough reflex (i.e., to stimuli distal to the anastomosis) and neurally mediated changes in airway bronchomotor tone. Airway reactivity does not appear to be increased, either at rest or in response to stimuli commonly encountered in the clinical setting.[418] Mucociliary clearance is also impaired in pulmonary allografts,[419] which together with immunosuppression and impaired cough, place the patient at increased risk for perioperative pneumonia. Interruption of lymphatic drainage imparts a susceptibility to increased pleural effusion and pulmonary edema and warrants increased attention to judicious fluid administration in the perioperative period. Hypoxic pulmonary vasoconstriction is intact in pulmonary allografts.[420]
Many considerations for the anesthesiologist are also presented by the pharmacologic regimen of immunosuppression. These do not generally differ between recipients of lungs or other organs because of close similarity of the drugs used for prevention of rejection.
|
|
|
|
|
|
|
|
|
|
|
|
|