|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Excellent descriptions of the surgical aspects of heart and heart-lung transplantation have been published,[299] [309] [310] but summary details of the orthotopic heart transplantation procedure are presented here with illustration in Figure 56-5A–B Figure 56-5C–E . For a description of the heart-lung transplantation procedure that is now performed fewer than 30 times per year in the United States, the reader should review the referenced surgical texts.[284] [285] [311]
After cannulation and readiness for bypass, CPB is initiated on arrival of the donor organ to the operating room. If used, the pulmonary artery catheter should be withdrawn into the superior vena cava (within its sterile sheath)
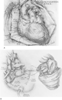
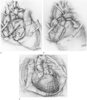 Figure 56-5
A, Preparation for aortic
and bicaval cannulation. Recipient cardiectomy follows initiation of cardiopulmonary
bypass. Ao, aorta; IVC, inferior vena cava; LV, left ventricle; PA, pulmonary artery;
RA, right atrium; RV, right ventricle; S-A, sinoatrial; SVC, superior vena cava.
B, Recipient cardiectomy is complete, with maximum
lengths of ascending aorta and main pulmonary artery left. Much of the recipient's
atria remain because they have been divided near the atrioventricular groove. Implantation
begins with the left atrial anastomosis. C, Right
atrial anastomosis.
D, Frequently, the pulmonary artery is next to be
anastomosed. However, as shown here, the aortic anastomosis may precede anastomosis
of the pulmonary artery to shorten the ischemic time of the graft. E,
The anastomoses have been completed and the patient has been separated from cardiopulmonary
bypass.(From Shumway SJ, Shumway NE:
Operative techniques in lung transplants. In Thoracic
Transplantation. Oxford, Blackwell Scientific, 1995, pp 164–167.)
Figure 56-5
A, Preparation for aortic
and bicaval cannulation. Recipient cardiectomy follows initiation of cardiopulmonary
bypass. Ao, aorta; IVC, inferior vena cava; LV, left ventricle; PA, pulmonary artery;
RA, right atrium; RV, right ventricle; S-A, sinoatrial; SVC, superior vena cava.
B, Recipient cardiectomy is complete, with maximum
lengths of ascending aorta and main pulmonary artery left. Much of the recipient's
atria remain because they have been divided near the atrioventricular groove. Implantation
begins with the left atrial anastomosis. C, Right
atrial anastomosis.
D, Frequently, the pulmonary artery is next to be
anastomosed. However, as shown here, the aortic anastomosis may precede anastomosis
of the pulmonary artery to shorten the ischemic time of the graft. E,
The anastomoses have been completed and the patient has been separated from cardiopulmonary
bypass.(From Shumway SJ, Shumway NE:
Operative techniques in lung transplants. In Thoracic
Transplantation. Oxford, Blackwell Scientific, 1995, pp 164–167.)
Until the time of reperfusion, the donor heart is cooled by submersion and irrigation with cold (4°C) crystalloid solution. The engraftment is begun with the donor left atrium, which is sutured to the recipient left atrial cuff. Subsequently, the right atrium is attached to the recipient counterpart. Importantly, the donor organ retains its sinoatrial node, and care is taken to avoid injury to this or other elements of the conduction system. This traditional ("biatrial") technique is often modified[312] [313] [314] [315] such that the heart is excised from the donor with the right atrium intact, and lengths of the donor's venae cavae are retained for subsequent anastomosis to the recipient venae cavae (the "bicaval" technique) ( Fig. 56-5A–E ).
Next, the great arteries of the donor heart are attached to those of the recipient. The ischemic time of the heart ends with removal of the aortic cross-clamp, at which point coronary reperfusion begins. The aorta may be anastomosed first to allow connection of the pulmonary artery to occur during coronary reperfusion. Technical considerations may, however, prompt anastomosis of the pulmonary artery before the aorta despite the need to minimize the ischemic interval.
Although timing of the donor operation imposes a limited interval for preoperative assessment, a focused review of the recipient's history and physical examination must be undertaken, with particular attention paid to the cause and nature of the cardiac dysfunction. Even though most patients have congestive heart failure from one cause or another, the specific etiologic factors (e.g., ischemic, idiopathic, or valvular pathology) should be understood insofar as they may have an impact on the prebypass anesthesia technique. A history of previous cardiac surgery is important because of its effect on the duration of surgery and technical factors, as well as the degree of blood loss and anticipated blood product administration. Recent exacerbations in symptoms and functional status should be elicited during the interview. The patient's medical regimen should be noted and is likely to reflect current strategies in the management of heart failure, CAD, or arrhythmia, including the use of β-blockers, diuretics, angiotensin-converting enzyme inhibitors, oral nitrates, calcium channel blockers, digoxin, amiodarone, and antiplatelet or anticoagulant therapy. Accordingly, serum electrolytes should be reviewed, along with recent coagulation studies and blood counts. Blood chemistry may otherwise reveal the effects of mild to moderate renal or hepatic dysfunction secondary to chronic hypoperfusion.
Those with specific indications may have implanted pacemakers, defibrillators, or even mechanical ventilatory or circulatory support. All such interventions should be reviewed and arrangements made for appropriate personnel to assist with their management (such as a cardiologist to deactivate an implanted cardioversion device or a perfusionist to operate a ventricular assist device). As with any unscheduled procedure, all other coexisting medical conditions or factors that may affect the conduct of anesthesia should be explored as necessary.
Relevant specialized investigations will have been performed preoperatively by the transplant team, and these studies should be reviewed by the anesthesiologist. The PVR should be noted, as well as the presence or absence of significant reversibility with vasodilator therapy. Although these findings will be of primary value to the anesthesiologist at the time of separation from CPB, these and other cardiac studies such as chest radiography, ECG, and echocardiography will also assist with the induction and maintenance of anesthesia before engraftment.
Preparation for anesthesia will be much the same as for cardiac operations in general. Special adjuncts such as nitric oxide (NO) delivery systems should be anticipated when appropriate and made available in the operating room. Typically, multiple immunosuppressive and anti-infective drugs will be required and delegated to the anesthesiologist preoperatively. The intended regimen, including dosages and timing, should be verified.
The timing of induction of anesthesia is important because the recipient's cardiectomy must proceed as soon as possible after arrival of the donor organ to the operating room. Close communication with the procurement team or transplant coordinator will permit estimation of the graft's arrival time. Refinements in organ preservation techniques aim to improve tolerance to ischemia and any preservation-related injury. It has been shown that optimal recovery of cardiac function may depend on minimizing the ischemic interval (i.e., time from application of the aortic cross-clamp at procurement to release of the cross-clamp during engraftment) to 4 hours or less. [285] To this end, the anesthesiologist and surgical team must anticipate the probable time required for induction of anesthesia, adequate exposure of the mediastinum, and readiness for CPB. This preparation time may be lengthened by previous cardiac surgery ("redo sternotomy") or the presence of an LV or RV assist device.
Anesthetic induction may be conducted by using principles similar to those followed for any patient with end-stage cardiac failure. Although sedation may be given before induction, it should be administered with the awareness that reliance on sympathetic vascular tone is high in most such patients. Anxiolytic medication is often postponed until arrival in the operating room. Vascular access procedures should be performed with proper aseptic technique because of the prominence of infection as a cause of postoperative morbidity and mortality in this immunocompromised population.[285] Venous access should be established and should include at least one large-bore cannula; two may be preferable, particularly when significant blood loss is anticipated, such as in those with previous sternotomies. Standard anesthetic monitoring is supplemented by invasive hemodynamic monitoring, including peripheral arterial,
Many patients who await transplantation receive intravenous inotropes (dobutamine and milrinone are frequent choices[316] ), intra-aortic balloon pumps, ventricular assist devices, or mechanical ventilation. [237] [299] [316] [317] [318] [319] [320] Induction of anesthesia in patients with either implantable or external ventricular assist devices can generally be achieved with conventional anesthetics with good hemodynamic stability.[321] [322] Although cardiac output will generally be quite stable by virtue of the assist device, standard principles of hemodynamic management otherwise apply to ensure adequate blood pressure and systemic perfusion. Adequate filling of the pump chambers in ventricular assist devices depends on maintenance of adequate circulating blood volume (preload), and mechanical ejection may be optimized by avoidance of increases in pulmonary or systemic vascular resistance (depending on which is the assisted ventricle).[321] Patients with one assisted ventricle are also vulnerable to factors that may impair function of the unassisted ventricle. For example, those with an assisted left ventricle may have marginal RV function and should therefore not be allowed to become fluid overloaded, nor should they be administered drugs that significantly impair myocardial contractility. Although mechanical support may confer tolerance to a variety of otherwise significant arrhythmias, those that preclude adequate ejection from the unassisted ventricle (e.g., ventricular fibrillation) must be immediately recognized and treated in standard fashion. Most, but not all commercially available assist devices require systemic anticoagulation to prevent device thrombosis, and anticoagulation should be continued in the operating room in accordance with the manufacturer's stated targets for indices of hemostasis (i.e., activated clotting time, prothrombin time, or partial thromboplastin time).
Other patients may not be supported by mechanical assistance, and many have borderline hemodynamic stability. Vasopressors or inotropes may be instituted in the operating room, before induction of anesthesia. Patients who are at the very limits of their hemodynamic compensatory mechanisms (e.g., systolic blood pressure <70 to 80 mm Hg, cardiac index <2 L/min/m2 ) may benefit from cannulation of femoral vessels before induction (after infiltration with a local anesthetic) and subsequent institution of extracorporeal membrane oxygenation (ECMO) or CPB support.[317] Extracorporeal circulation may be continued via the femoral vessels throughout induction, sternotomy, and readiness for cardiectomy.
The potential for aspiration of gastric contents should be considered because of the unscheduled nature of the procedure. Accepted methods should be used to neutralize and minimize gastric contents and to avoid gastroesophageal reflux, all within the confines of a carefully titrated induction. Some liberalization of the rapid-sequence intubation technique will probably be required. Generally, induction agents should be chosen that do not cause significant myocardial depression. Many variations of drugs and doses have been used with success. Opioids such as sufentanil, 0.5 to 2.0 µg/kg, or fentanyl, 7 to 15 µg/kg, may be combined with benzodiazepines such as midazolam, 0.05 to 0.1 mg/kg, or etomidate, 0.2 to 0.3 mg/kg.[323] Alternatively, an opioid may be administered in higher dose as a sole induction agent.[322] Although it exhibits some contractility-reducing properties, ketamine, 0.5 to 1.5 mg/kg, has been used with good success in cardiac transplantation, including patients with Eisenmenger's syndrome (right-to-left shunting across a cardiac defect because of elevated PVR) who are undergoing heart-lung transplantation. [324] The required dose of any drug may vary widely and should be titrated according to the hemodynamics and depth of anesthesia. Hypotension may ensue regardless of the specific drugs used because of a reduction in preload or afterload as sympathetic tone is mitigated by the onset of anesthesia. Vascular tone may be maintained by the continuous infusion or intermittent administration of drugs with α-agonist properties such as phenylephrine or norepinephrine.
The heart rate should be maintained close to baseline levels; tachycardia may provoke myocardial ischemia in susceptible patients, whereas bradycardia will reduce cardiac output because of the low, fixed stroke volume that is characteristic of dilated cardiomyopathy. The onset of apnea with induction of anesthesia should be met with the careful delivery of positive-pressure ventilation. Undue positive pleural pressure may reduce venous return and essential myocardial preload. In contrast, hypoventilation with associated hypercapnia may impair LV filling by means of increased PVR and RV failure. Endotracheal intubation is facilitated with the use of a neuromuscular blocking agent that is devoid of significant histamine-releasing properties, such as pancuronium or, should concerns of airway or aspiration prevail, succinylcholine.
Surgical events before the initiation of CPB provide comparatively few obstacles to hemodynamic stability, provided that intravascular volume is carefully maintained along with myocardial contractility and systemic vascular resistance. Balanced anesthesia with the continuous infusion or intermittent bolus administration of opioids, benzodiazepines, and muscle relaxants is typical and may be supplemented with low-dose volatile anesthetics (e.g., <0.4% isoflurane). Synthetic opioids have been used as sole anesthetics, with the total fentanyl or sufentanil dosage often exceeding 80 or 20 µg/kg, respectively.[322] Concern regarding intraoperative awareness and delayed extubation has led to widespread use of balanced techniques. Nitrous oxide is not contraindicated; however, it may contribute to the expansion of intracardiac or intravascular air in the postbypass period and may have deleterious effects on PVR.
Antifibrinolytics are known to reduce blood loss and blood product administration in cardiac surgery with CPB.[325] [326] [327] [328] [329] [330] [331] [332] [333] [334] [335] [336] [337] Many patients presenting for heart transplantation receive antiplatelet or anticoagulant therapy for
Most of the remaining discussion deals with the common orthotopic heart transplantation procedure. After aortic anastomosis, measures are taken to evacuate air from the heart before removal of the cross-clamp and cardiac ejection. Transesophageal echocardiography (TEE) may be of assistance with the detection of significant unrecognized foci of air within the left ventricle (typically located anteriorly, near the apex). Myocardial reperfusion commences at the time of aortic cross-clamp removal. Typical immunosuppression regimens call for the administration of intravenous corticosteroid immediately before reperfusion, which may be delegated to the anesthesiologist.
As with cardiac surgery in general, the anesthetic goals at the time of separation from CPB include the provision of optimal conditions for separation and the anticipation of probable hemodynamic derangements. Generally, reperfusion and rewarming are followed by resumption of normal sinus rhythm. With a relative bradycardia or atrioventricular conduction block, adequate rate and rhythm may be achieved by the infusion of a chronotropic agent (such as isoproterenol or dobutamine) or the use of a temporary pacemaker.[339] [340] An appropriate target heart rate for weaning from CPB may be in the range of 90 to 110 beats/min.[299] [301]
Because of the lack of autonomic innervation, only direct-acting sympathomimetics such as catecholamines or phosphodiesterase inhibitors can be used for inotropic or chronotropic effect. Drugs that increase the heart rate with a vagolytic effect, such as atropine or other antimuscarinics, are ineffective. Autonomic reflexes that normally serve to modulate cardiac activity, such as the tachycardic response to hypovolemia, hypotension, or light anesthesia or the bradycardic response to laryngoscopy or visceral stimulation, are absent in denervated hearts. Accordingly, they cannot be relied on to compensate for such physiologic stresses or to warn the anesthesiologist of their occurrence. (Partial autonomic reinnervation has been observed in patients studied after transplantation,[341] [342] [343] [344] but this is a late phenomenon of uncertain clinical significance.)
Most grafts ultimately recover with normal ventricular function. The period of separation from CPB, however, may be complicated by some degree of myocardial dysfunction that is often attributable to incomplete or prolonged preservation (i.e., ischemia) or related to circumstances of the critical illness of the donor. Although LV function is generally adequate, RV function is of particular concern after heart transplantation and is clearly of major importance to cardiac output, hemodynamic stability, and end-organ perfusion. Even though it is not possible to predict all cases of acute right heart failure, recipients with elevated preoperative PVR greater than 4 Wood units are at highest risk for this complication. This is particularly evident when reversibility has not been demonstrable with the use of pulmonary vasodilating drugs.[345] Even an intrinsically normal donor heart without significant ischemic insult is likely to become dysfunctional if acutely challenged by elevated RV afterload.
Recognition of RV failure is facilitated by the detection of typical patterns of central pressure derangement in the context of low cardiac output, as determined by thermodilution, or systemic hypotension. Typically, CVP is elevated above what is necessary for adequate RV preload (i.e., greater than 15 mm Hg), along with elevated PAP (i.e., mean PAP >40 mm Hg or greater than three quarters of mean arterial pressure). PCWP may be normal or low as a result of decreased LV filling, yet decreased LV compliance is typical and may preclude straightforward inference of LV preload from PCWP. TEE may be of particular importance in this setting[345] [346] and can suggest acute RV failure by way of right atrial and ventricular enlargement along with decreased RV systolic function. A mild to moderate degree of tricuspid regurgitation is typical, but it may be severe in patients with acute RV failure. Both the interatrial septum and the interventricular septum may be deflected toward the left because of volume and pressure overload of the right cardiac chambers. Evaluation of the left ventricle may reveal underfilling, thereby accounting for poor stroke volume in the face of normal or even hyperdynamic LV systolic function.
Although anticipation and active prevention of RV failure may
be the optimal strategy,[345]
such is clearly not
always possible. Certain principles are generally observed when attempting to forestall
or treat the development of RV failure ( Table
56-3
). Inotropic support is usually applied proactively at the time of
separation from CPB, which benefits both LV and RV function. Some inotropic agents,
such as milrinone and dobutamine, are notable for their ability to decrease PVR and
are thus frequently used in this setting. Isoproterenol shares these properties
and
| 1. Optimize preload | Avoid RV distention and underfilling |
| 2. Inotropic and chronotropic support | Isoproterenol, milrinone, dobutamine |
| 3. Maintain coronary perfusion | Vasopressors, avoid RV distention |
| 4. Conservative measures to lower PVR | High FIO2 , avoid hypercapnia and hypothermia, optimize lung volume and airway pressure |
| 5. Pharmacologically lower PVR | Nitrates, PGE1 , prostacyclin, inhaled NO |
| 6. Mechanical support | RV assist device, intra-aortic balloon pump |
| NO, nitric oxide; PGE1 , prostaglandin E1 ; PVR, pulmonary vascular resistance; RV, right ventricular. | |
Particular attention should be paid to volume management and RV loading conditions. Although adequate RV preload must be maintained to optimize cardiac output, excessive loading is unlikely to improve stroke volume and may actually worsen systolic function, create significant tricuspid regurgitation, and aggravate RV ischemia. CVP and TEE serve as useful adjuncts to guide volume management.
The persistence of significant pulmonary artery hypertension or RV failure despite such maneuvers may warrant the administration of other pharmacologic drugs with pulmonary vasodilating properties. Nitroglycerin, nitroprusside, prostacyclin, and prostaglandin E1 have all been used to this end, but in each case an α1 -agonist may be required to counter their nonspecific vasodilating effect on the pulmonary and systemic vasculature.[323] [345] The development of commercially available technology able to deliver inhaled NO safely and easily in the operating room has led to its application in various intraoperative settings. When administered by the inhalation route, NO creates localized relaxation of pulmonary blood vessels yet becomes rapidly inactivated by circulating hemoglobin, thereby minimizing its exposure to the systemic vasculature. NO has been used in acute RV failure after heart transplantation with salutary effects on PVR, CVP, and cardiac output[347] [348] [349] [350] [351] [352] and neutral effects on SVR and mean arterial pressure, which may indeed forestall the need for more aggressive support such as placement of an RV assist device.[345] [348] Inhaled NO may be used proactively in patients at risk for RV failure on separation from CPB[348] [349] or be used more selectively in patients in whom pulmonary hypertension or RV dysfunction subsequently develops despite optimal hemodynamic management with intravenous therapy.[347] [353] Novel drugs in the treatment of pulmonary hypertension, such as sildenafil and bosentan, have shown promise in patients outside the setting of heart transplantation,[354] [355] [356] [357] [358] but they have not, at the time of this writing, been reported in cardiac surgery.
Nonpharmacologic measures to minimize PVR include the avoidance of hypercapnia, delivery of high FIO2 , and maintenance of normothermia. The ventilatory strategy should minimize significant atelectasis while avoiding unnecessary increases in peak or mean airway pressure, either of which can contribute to pulmonary microvascular collapse and elevation of PVR.
In cases of refractory RV failure, the use of an RV assist device may restore acceptable hemodynamics and systemic perfusion during the postoperative period.[345] [348] Underlying the use of this device is the belief that PVR may be expected to normalize in the ensuing hours or days after cardiac transplantation,[359] in part because of regression of left atrial pressure and clearance of transfusion- and CPB-related inflammatory mediators. The right ventricle may also adapt to elevated PVR in the postoperative period, which will allow successful withdrawal of support with the RV assist device, even in cases of incomplete normalization of the pulmonary vasculature.[346]
Though of characteristic concern in heart transplantation, RV failure is not the only source of instability in the post-CPB period. Arrhythmia, hypovolemia, LV dysfunction, and even anastomotic obstruction may contribute to hemodynamic compromise and must be corrected. TEE is useful for assessment of valvular and LV function after separation, as well as for evaluation of vascular connections.[360] Rarely, obstruction occurs at the atrioventricular valves because of redundant native atrial tissue at the site of anastomosis with the graft.[361]
Although acute rejection is typically manifested in the early postoperative period or beyond, hyperacute rejection is an uncommon, yet critical development that occurs shortly after reperfusion of the donor heart. It is caused by preformed antibodies to donor antigen, which may be the result of previous blood transfusions, pregnancy, or inadvertent ABO blood group incompatibility.[311] [362] This catastrophic event is characterized by cyanosis, severe biventricular dysfunction, and loss of the graft. A ventricular assist device may provide hope for temporization pending the availability of a second organ for transplantation.
Early postoperative care occurs in an ICU setting, commonly in isolated rooms to minimize exposure to infective agents.[363] Infection—particularly pneumonia—continues to be one of the most important causes of early (within 30 days) mortality in thoracic organ transplantation.[285] As with all critical care patients, fever is aggressively investigated and treated with empirical antibiotics pending the results of blood, urine, and sputum microbiology.
Mechanical ventilation is continued and weaned within 1 to 2 days in the absence of significant hemodynamic or other complications. Although normal or near-normal biventricular function is expected in most cases, RV dysfunction may develop and persist because of increased PVR. NO, intravenous inotropes and vasodilators, or ventricular assist devices may be continued or initiated postoperatively, as discussed in the preceding section.
Renal function may have been compromised by chronic hypoperfusion before transplantation. Perioperative hemodynamic instability may combine with commonly used nephrotoxic drugs, such as cyclosporine or aminoglycoside antibiotics, to threaten renal function in the postoperative period. Loop diuretics may assist in the augmentation of urine output and facilitate volume management, yet renal function may best be defended by the optimization of hemodynamic management and cardiac output.
Early postoperative survival is threatened by acute (cellular) rejection. Surveillance endomyocardial biopsies
Another postoperative challenge is mediastinal bleeding, which may persist from the operating room. It frequently prompts re-sternotomy and surgical exploration if it cannot be abated by blood component therapy directed toward laboratory-defined coagulation defects. Bradycardia from sinoatrial node dysfunction (or less commonly, atrioventricular node dysfunction) may necessitate the use of chronotropic drugs such as isoproterenol or temporary epicardial pacing for varying intervals of up to 3 weeks. In a minority of these cases, or about 4% to 7% overall, patients do not recover adequate chronotropic function, and permanent pacemaker implantation is required.[339] [340] [363] Systemic hypertension frequently develops in the early postoperative period and may be at least partly due to the administration of cyclosporine.
Despite the improved quality and duration of life in heart transplant recipients, there are still specific transplantation- and medication-related morbidities that characteristically develop over time ( Table 56-4 ). These morbidities, in conjunction with altered cardiac physiology, provide the anesthesiologist with a number of issues that must be considered before the provision of anesthesia for noncardiac surgery.
Many of the conditions that develop or worsen after cardiac transplantation
are related to the medications currently used for immunosuppression, and these are
discussed further in the later section "Immunosuppression." The long-term use of
prednisone probably contributes to the development of hyperlipidemia, hypertension,
and diabetes, whereas cyclosporine impairs renal function
| Condition | Incidence 5 Years Post-transplant |
|---|---|
| Hypertension | 95.1% |
| Renal dysfunction | 30.9% |
| Hyperlipidemia | 81.3% |
| Diabetes | 32.0% |
| Coronary artery vasculopathy | 33.2% |
| Malignancy | 29% at 7 years |
When assessed at 5 years, coronary artery vasculopathy (CAV) has developed in about 33% of heart transplant recipients.[285] This form of ischemic heart disease most likely evolves from chronic immune-mediated injury, but it may also be aggravated by transplantation factors (such as prolonged ischemia), smoking, hypertension, hyperglycemia, or hyperlipidemia.[286] Typically, patients receive 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (e.g., pravastatin), calcium channel blockers (e.g., diltiazem), or other antihypertensive medications, not only to treat disorders of lipid metabolism or hypertension but also for their preventive effects against CAV.[287] Although angiography and intravascular ultrasound provide a means for annual surveillance, DSE may provide a better assessment of the impact of CAV on myocardial perfusion and function and may thus be a better predictor of subsequent cardiac events.[286] Regional or even global myocardial dysfunction may develop in advanced cases. Patients with significant CAV may experience dyspnea on exertion instead of angina because of their lack of autonomic innervation.[286] [323] As ischemic damage to the graft progresses, signs and symptoms of congestive heart failure typically occur and are ultimately followed by death or retransplantation. Recent cardiac studies from the patient's transplant center are generally available for review, but any patient with concerning signs or symptoms should undergo additional noninvasive testing, such as ECG and echocardiography.[286] If patients experience a decline in their functional status, acute rejection must be considered. Acute rejection is most likely if it occurs within the first year after transplantation or is accompanied by fever or malaise. Before all nonemergency, noncardiac surgeries, any patient with acute rejection should have a definitive diagnosis, that is, one obtained from an endomyocardial biopsy. This episode should then be treated appropriately before the surgery.
In most cases, cardiac transplant patients may safely undergo general or regional anesthesia.[365] No specific technique or selection of anesthetics has proved to be to be superior in the intraoperative conduct of anesthesia, although renal dysfunction may necessitate the exclusion or dose adjustment of certain nephrotoxic medications and other drugs that are eliminated by the kidneys. Unless the patient has been successfully weaned from glucocorticoids, supplemental perioperative steroid administration should be considered. Depending on the surgery planned, monitoring for myocardial ischemia should include either multilead ECG or TEE. As with all
As described previously, drugs that normally alter the heart rate or nodal function by indirect mechanisms (e.g., antimuscarinics, anticholinesterases, pancuronium, digoxin) will lack such effects. The absence of cardiac vagal tone will result in a baseline heart rate of 90 to 100 beats/min in most patients. It will cause loss of the bradycardic response to such stimuli as laryngoscopy, hypertension, and carotid sinus massage. Drugs with direct effects at cardiac autonomic receptors, such as isoproterenol and epinephrine, should be selected for inotropic or chronotropic therapy. As with cardiac sympathetic innervation, limited vagal reinnervation may develop over years after transplantation.[341]
|
|
|
|
|
|
|
|
|
|
|
|
|