|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Airway receptors that may be influenced by inhaled anesthetics include laryngeal and pulmonary irritant receptors and pulmonary stretch receptors. It has been suggested
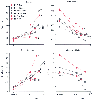 Figure 6-13
Comparison of mean changes in resting PaCO2
,
tidal volume, respiratory rate, and minute ventilation in patients anesthetized with
halothane (H), isoflurane (I), enflurane (E), sevoflurane (S), desflurane (D), or
nitrous oxide (N). Anesthetic-induced tachypnea compensates in part for the ventilatory
depression caused by all volatile anesthetics (i.e., decreased minute ventilation
and tidal volume and concomitant increased PaCO2
).
Desflurane results in the greatest increase in PaCO2
,
with corresponding reductions in tidal volume and minute ventilation. Isoflurane,
like all other inhaled agents, increases respiratory rate, but isoflurane does not
result in dose-dependent tachypnea. (Data from references [224]
[225]
[226]
[227]
[231]
[232]
.)
Figure 6-13
Comparison of mean changes in resting PaCO2
,
tidal volume, respiratory rate, and minute ventilation in patients anesthetized with
halothane (H), isoflurane (I), enflurane (E), sevoflurane (S), desflurane (D), or
nitrous oxide (N). Anesthetic-induced tachypnea compensates in part for the ventilatory
depression caused by all volatile anesthetics (i.e., decreased minute ventilation
and tidal volume and concomitant increased PaCO2
).
Desflurane results in the greatest increase in PaCO2
,
with corresponding reductions in tidal volume and minute ventilation. Isoflurane,
like all other inhaled agents, increases respiratory rate, but isoflurane does not
result in dose-dependent tachypnea. (Data from references [224]
[225]
[226]
[227]
[231]
[232]
.)
 Figure 6-14
The effect of surgical stimulation on the ventilatory
depression of inhaled anesthesia with isoflurane in the presence and absence of nitrous
oxide. Surgical stimulation increased alveolar ventilation and decreased PaCO2
at all depths of anesthesia examined. (Adapted Eger EI, Dolan WM, Stevens
WC, et al: Surgical stimulation antagonizes the respiratory depression produced
by Forane. Anesthesiology 36:544, 1972.)
Figure 6-14
The effect of surgical stimulation on the ventilatory
depression of inhaled anesthesia with isoflurane in the presence and absence of nitrous
oxide. Surgical stimulation increased alveolar ventilation and decreased PaCO2
at all depths of anesthesia examined. (Adapted Eger EI, Dolan WM, Stevens
WC, et al: Surgical stimulation antagonizes the respiratory depression produced
by Forane. Anesthesiology 36:544, 1972.)
Slowly adapting pulmonary stretch receptors are located within small airway smooth muscle and respond to stretching or changes in lung volume. A particularly high concentration of these receptors is located at the carina. Increases in lung volume enhance afferent nerve traffic through the vagus nerve to the respiratory control center and thereby inhibit further inspiration. This phenomenon is known as the Hering-Breuer reflex and appears to be independent of flecainide-sensitive Na+ channels or 4-aminopyridine-sensitive K+ channels.[252] The limitation of inspiration elicited by pulmonary stretch receptors may determine the relationship between tidal volume and respiratory frequency in experimental animals, but the Hering-Breuer reflex cannot be demonstrated in the awake, resting human during normal tidal volume breathing. Activation of slow-adapting pulmonary stretch receptors by persistent elevations in end-expiratory lung volumes produced an inhibitory effect on central drive (i.e., an increase in apneic threshold) and an attenuated ventilatory response to progressive hypercapnia.[253] The alteration in ventilatory pattern caused by anesthetics has been attributed to relative sensitization of pulmonary stretch receptors that subsequently produce tachypnea and low tidal volumes. The presence of a volatile anesthetic increased vagal afferent discharge at various lung volumes in decerebrate cats (i.e., sensitization of pulmonary stretch receptors),[254] but little evidence exists of such a mechanism in humans.[255] There is evidence in the cat that halothane-induced tachypnea is primarily a suprapontine effect, but the mechanism of production of tachypnea with decreased tidal volume in anesthetized humans continues to remain unclear.
The direct effects of halothane, isoflurane, and enflurane on pulmonary and laryngeal irritant receptors and tracheobronchial slow-adapting stretch receptors have been investigated in spontaneously breathing and vagotomized, paralyzed dogs.[256] [257] Several types of afferent receptors in the airways have been identified and include pressure, drive, cold, irritant, slowly and rapidly adapting stretch receptors, and C-fibers.[258] Rapidly adapting receptors occur throughout the respiratory tract from the nose to the bronchi. These receptors are characterized by an irregular discharge pattern and rapid adaptation to a stable volume stimulus, but they also display a more gradual adaptation to a chemical stimulus. Rapidly adapting laryngeal (irritant) receptors are most likely responsible for coughing, mucus secretion, and expiratory reflexes including bronchial and laryngeal constriction. Rapidly adapting receptors in the bronchi are more chemosensitive than those located in the more proximal airway but can also cause coughing, mucus secretion, bronchocon-striction, and laryngospasm. Rapidly adapting receptors in the bronchi stimulate hyperventilation.[259]
Volatile anesthetics increase the activity of laryngeal irritant receptors[256] and inhibit pulmonary irritant receptors. [257] Volatile anesthetics also elevate the excitation threshold and increase the sensitivity of low-threshold stretch receptors. Halothane and, to a lesser extent, sevoflurane attenuate end-expiratory discharge frequency of slow-adapting pulmonary receptors.[257] [260] These agents may not affect[260] or even augment [257] the inspiratory activity. The clinical implications of these findings have yet to be precisely determined, but these anesthetic-induced changes in slow-adapting receptor function may play a role in the reduction of bronchomotor tone observed with volatile agents.
It has been suggested that volatile anesthesia may result in genioglossus muscle relaxation, causing posterior tongue displacement and consequent upper airway obstruction. However, this hypothesis has not been confirmed.[261] [262] [263] Anteroposterior displacement of upper airway structures occurs with changes in head position that occur in the same direction as the mandible. Administration of a volatile anesthetic and a neuromuscular blocker may widen the dimensions of the larynx,[261] but the nasopharyngeal airway decreases in size. During propofol sedation in children, the shape of the upper airway is oblong, and the anterior-posterior diameter is greater than the transverse diameter. This relationship reverses on awakening.[263] Patency of the upper airway during awakening may depend on contraction of pharyngeal dilating muscles that temporally precede diaphragmatic contraction.[264] The increase in thoracic inspiratory activity may also produce caudal traction on the upper airway and assist in expanding the upper airway during inspiration.[265] Central output to the genioglossus muscle and local reflex-mediated activation are important in maintaining upper airway patency,
Volatile anesthetics produce a greater depression of the upper airway electromyogram[268] or nerve activity[269] compared with diaphragmatic activity in anesthetized, spontaneously breathing cats [268] ( Fig. 6-15 ) and paralyzed, ventilated, vagotomized cats.[269] The extent to which this depression of upper airway motor neuron activity occurs as a result of anesthetic-induced inhibition of the reticular activating system is unknown.
The severity of airway hyperreactivity was determined in 123 children breathing through LMAs and receiving isoflurane or sevoflurane anesthesia.[270] Isoflurane was associated with greater airway hyperreactivity than was sevoflurane during LMA removal. The depth of anesthesia during LMA removal did not affect the severity or incidence of airway hyperreactivity during administration of sevoflurane, but LMA removal in the presence of isoflurane was associated with more adverse airway events. Sevoflurane (1 MAC) also provides greater attenuation of moderate to severe responses to tracheal stimulation by inflating the endotracheal tube cuff compared with desflurane.[271] However, higher concentrations (1.8 MAC) of these agents produced similar attenuation of coughing, tachycardia, and hypertension associated with tracheal stimulation.
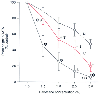 Figure 6-15
Decrease in phasic inspiratory muscle activity, expressed
as the peak height of moving time average (MTA), in percent change from control (1%
halothane), during halothane anesthesia in adult cats. Values are expressed as the
mean ± SEM. The middle curve represents the intercostal muscle (IC) (*P
< .05 compared with the diaphragm [DI]; **P
< .05 compared with the genioglossus muscle [GG]). Notice the differential sensitivities
of these respiratory muscles. (From Ochiai R, Guthrie RD, Motoyama EK:
Effects of varying concentrations of halothane on the activity of the genioglossus,
intercostals, and diaphragm in cats: an electromyographic study. Anesthesiology
70:812, 1989.)
Figure 6-15
Decrease in phasic inspiratory muscle activity, expressed
as the peak height of moving time average (MTA), in percent change from control (1%
halothane), during halothane anesthesia in adult cats. Values are expressed as the
mean ± SEM. The middle curve represents the intercostal muscle (IC) (*P
< .05 compared with the diaphragm [DI]; **P
< .05 compared with the genioglossus muscle [GG]). Notice the differential sensitivities
of these respiratory muscles. (From Ochiai R, Guthrie RD, Motoyama EK:
Effects of varying concentrations of halothane on the activity of the genioglossus,
intercostals, and diaphragm in cats: an electromyographic study. Anesthesiology
70:812, 1989.)
|
|
|
|
|
|
|
|
|
|
|
|
|