|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The anesthesiologist's first "interface" with a patient who has
sustained a traumatic brain injury (TBI) may come as a result of a request for assistance
with airway management. It has been determined empirically that patients with Glasgow
Coma Scale (GCS) scores of 7 to 8 ( Table
53-10
) or less will eventually require intubation and controlled ventilation
for ICP or airway control (or for both). Accordingly,
| Eyes open | Never | 1 |
|
|
To pain | 2 |
|
|
To speech | 3 |
|
|
Spontaneously | 4 |
| Best verbal responses | None | 1 |
|
|
Garbled/incomprehensible sounds | 2 |
|
|
Inappropriate words | 3 |
|
|
Confused but converses | 4 |
|
|
Oriented | 5 |
| Best motor responses | None | 1 |
|
|
Extension (decerebrate rigidity) | 2 |
|
|
Abnormal flexion (decorticate rigidity) | 3 |
|
|
Withdrawal | 4 |
|
|
Localizes pain | 5 |
|
|
Obeys commands | 6 |
| Total |
|
3–15 |
The possibility of causing or aggravating an injury to the cervical
spine is a relevant concern. Approximately 2% of patients with a closed-head injury
who survive to reach a hospital will have a fracture of the cervical spine.[247]
Somewhat surprisingly, the incidence of cervical spine injury is similar (1.8% to
6.0%) for all blunt trauma victims with or without an associated TBI.[247]
[248]
[249]
[250]
This incidence suggests that a "hypnotic-relaxant-direct laryngoscopy" approach
for all patients with a closed-head injury might convey a measurable risk of injuring
the cervical cord. Nonetheless, although the literature contains contradictions,
several published series have concluded that rapidsequence induction does not convey
a significant risk of neurologic injury.[250]
[251]
[252]
[253]
[254]
However, it is possible that the incidence of intubation-related neurologic injury
is underreported. An informal survey by Criswell and colleagues[250]
indicated that there have been more such events than one can infer from the published
literature.[255]
[256]
Nonetheless, the literature argues that we will "get away with it" most of the time,
and there are certainly units in which most patients requiring airway control are
intubated with the use of a hypnotic-relaxant-direct laryngoscopy sequence.
| Full stomach |
| Uncertain cervical spine |
| Uncertain airway |
| Blood |
| Airway injury (larynx, cricoarytenoid cartilage) |
| Skull base fracture |
| Uncertain volume status |
| Uncooperative/combative |
| Hypoxemia |
| Increased intracranial pressure |
When a hypnotic-relaxant sequence is used, the standard approach includes the use of cricoid pressure and in-line axial stabilization. In-line traction was once favored but has been supplanted by stabilization because of the perceived risk of overdistraction and cord injury in the event of gross instability. The largest of the clinical series in which it was concluded that oral intubation with anesthesia and relaxation is reasonable[251] used in-line stabilization with the patient's occiput held firmly on the backboard, thus limiting the amount of "sniff" that was feasible ( Fig. 53-15 ). There is no question that in-line stabilization, properly performed, will make laryngoscopy somewhat more difficult. However, it serves to decrease the amount of atlanto-occipital extension necessary to achieve visualization of the glottis, [258] probably because performing laryngoscopy against the assistant's counter-pressure results in greater compression of the soft tissue structures of the tongue and floor of mouth. Some recommend leaving the back half of the Philadelphia collar
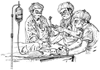 Figure 53-15
Intubating an acute trauma patient with an uncertain
cervical spine. A hypnotic and a relaxant have been administered. One assistant
maintains in-line axial stabilization with the occiput held firmly to the backboard;
a second applies cricoid pressure. The posterior portion of the cervical collar
remains in place to "discourage" atlanto-axial extension. (From Stene JD:
Anesthesia for the critically ill trauma patient. In
Siegel JH [ed]: Trauma: Emergency Surgery and Critical Care. Melbourne, Australia,
Churchill Livingstone, 1987, p 843.)
Figure 53-15
Intubating an acute trauma patient with an uncertain
cervical spine. A hypnotic and a relaxant have been administered. One assistant
maintains in-line axial stabilization with the occiput held firmly to the backboard;
a second applies cricoid pressure. The posterior portion of the cervical collar
remains in place to "discourage" atlanto-axial extension. (From Stene JD:
Anesthesia for the critically ill trauma patient. In
Siegel JH [ed]: Trauma: Emergency Surgery and Critical Care. Melbourne, Australia,
Churchill Livingstone, 1987, p 843.)
In the resuscitation situation, before initiating a hypnotic-relaxant sequence, the anesthesiologist should confirm the availability of both cricothyrotomy equipment and someone to make immediate, skilled use of it if necessary. A recently injured brain is very intolerant of hypoxia and hypotension.[259] It is inevitable that there will be the occasional failed intubation. In the extensive experience of the Cowley Shock-Trauma Center in Baltimore, the cricothyrotomy rate is 0.3% (personal communication, Colin Mackenzie, M.D.). As is the case in many other situations, the laryngeal mask airway (LMA) may be a very useful device for temporizing in the face of a failed intubation and may also provide access for intubation as an alternative to cricothyrotomy.
As noted in Chapter 21 , although succinylcholine can cause increases in ICP, these increments are small and probably do not, in fact, occur at all in patients with serious cerebral injuries.[6] Accordingly, succinylcholine should not be viewed as contraindicated in a TBI victim. If and when there is an urgent need to secure the airway (to control carbon dioxide tension and guarantee oxygenation) and if succinylcholine is in other respects the appropriate drug to achieve that end, it should be used.
In two clinical surveys, alert, nonintoxicated patients with a cervical spine fracture invariably had pain, tenderness, or neurologic signs.[248] [260] Accordingly, in spite of the frequency with which you will meet patients still wearing their Philadelphia collars because the neck has not yet been "cleared," no special precautions appear to be warranted in an asymptomatic, alert patient. Note also that if the clinical situation or examination is suggestive of a cervical spine injury, a normal lateral radiograph (the anteroposterior and through-the-mouth odontoid views are frequently not taken during the initial evaluation) cannot provide complete reassurance. The lateral view has been reported to miss between 15%[261] and 26%[248] of fractures.
Craniotomies will most commonly be performed for the evacuation of subdural, epidural, or intracerebral hematomas. The anesthetic approach is similar for all three. The guiding principles have been discussed in the section "Control of Intracranial Pressure/Brain Relaxation." In general, anesthetics that are known to be cerebral vasoconstrictors will be preferable to those that have the potential to dilate the cerebral circulation. All of the intravenous anesthetics, except perhaps ketamine, cause some cerebral vasoconstriction and are reasonable choices, provided that they are consistent with hemodynamic stability. All of the inhaled anesthetics (N2 O and all of the vapors) have some cerebral vasodilatory effect. Although their administration will frequently be consistent with acceptable ICP levels or appropriate conditions in the surgical field (or both), when ICP is out of control (or unknown) or the surgical field is "tight," eliminating the inhaled anesthetics in favor of fixed anesthetics is appropriate. For patients who are likely to remain tracheally intubated postoperatively, an anesthetic based primarily
The anesthesiologist should appreciate that the priority is to open the cranium as rapidly as possible. After achieving intravenous access, the craniotomy should never be delayed significantly by line placement. An arterial line, often placed after induction in urgent situations, is appropriate for essentially all acute trauma craniotomies. The decision to achieve central venous access can be based on the patient's hemodynamic status. Infrequently, management of a depressed skull fracture over the sagittal sinus will justify precordial Doppler examination and, subject to the surgeon's opinion of the risk for VAE, a right heart catheter.
The concept that the injured brain is extremely vulnerable to what would otherwise be a minor insult, for example, modest hypotension or moderate hypoxia, has been well confirmed in the laboratory.[262] [263] [264] Although as yet no completely conclusive data have been derived from humans, several clinical surveys are strongly supportive of the adverse effect of minor degrees of hypotension in the post-TBI period.[139] [259] [265] [266] [267] The explanation for this vulnerability to hypotension probably resides in part in the observation that some patients in the postinjury period have regions of brain with precariously low CBF[28] [38] [268] in which autoregulation may also be defective. [42] [269] In addition, ample evidence indicates that the low postinsult CBF values correlate with a poor eventual outcome[28] [41] [266] [270] and that a large percentage of patients who die after TBI have pathologic changes consistent with ischemia.[156] These observations have resulted in a much greater emphasis by many neurosurgeons and neurointensivists on aggressive support of blood pressure in TBI patients.
What constitutes an appropriate blood pressure? Systematic studies, in particular, those conducted at the University of Edinburgh, have revealed evidence that indices of the adequacy of cerebral perfusion derived from SjVO2 and transcranial Doppler data begin to deteriorate below a mean CPP of 70 mm Hg. [266] [271] [272] (Recall that CPP = MAP − ICP.) As a result, many neurosurgical groups adopted 70 mm Hg as the target CPP.[268] [269] [272] An expert panel[273] found the data insufficient to justify establishing 70 mm Hg as a "standard" CPP target, but instead identified it as a reasonable management "option," and other groups and authorities have adopted a CPP of 60 mm Hg as the management target.[274] [275] [276]
Added to this discussion are two alternative opinions regarding blood pressure management ( Fig. 53-16 ). The first, promoted by the neurosurgery group at the University of
 Figure 53-16
Relationship of cerebral blood flow (CBF) to blood pressure
after head injury. There are three cerebral perfusion pressure (CPP) management
strategies (see text) driven by differing beliefs about common pathophysiologic derangements.
The most commonly held, the "Edinburgh" (so named for the institution of the original
proponents), emphasizes low postinjury CBF, impaired autoregulation, and the necessity
to support CPP (mean arterial pressure [MAP] — intracranial pressure [ICP])
to 70 mm Hg. The "Lund concept" emphasizes the contribution of hyperemia to the
occurrence of elevated ICP. That approach uses antihypertensive agents to reduce
blood pressure while maintaining CPP over 50 mm Hg.[277]
That CPP target has increased, in the most recent iteration to 60 to 70 mm Hg with
allowance for occasional reduction to 50.[278]
An approach emanating from the University of Alabama, identified as the "Birmingham"
concept, entails pharmacologically induced hypertension. This approach is based
on the belief that autoregulation is largely intact and that hypertension will result
in cerebral vasoconstriction with concomitantly reduced CBV and ICP.[279]
[280]
Figure 53-16
Relationship of cerebral blood flow (CBF) to blood pressure
after head injury. There are three cerebral perfusion pressure (CPP) management
strategies (see text) driven by differing beliefs about common pathophysiologic derangements.
The most commonly held, the "Edinburgh" (so named for the institution of the original
proponents), emphasizes low postinjury CBF, impaired autoregulation, and the necessity
to support CPP (mean arterial pressure [MAP] — intracranial pressure [ICP])
to 70 mm Hg. The "Lund concept" emphasizes the contribution of hyperemia to the
occurrence of elevated ICP. That approach uses antihypertensive agents to reduce
blood pressure while maintaining CPP over 50 mm Hg.[277]
That CPP target has increased, in the most recent iteration to 60 to 70 mm Hg with
allowance for occasional reduction to 50.[278]
An approach emanating from the University of Alabama, identified as the "Birmingham"
concept, entails pharmacologically induced hypertension. This approach is based
on the belief that autoregulation is largely intact and that hypertension will result
in cerebral vasoconstriction with concomitantly reduced CBV and ICP.[279]
[280]
What is an anesthesiologist managing a TBI patient to do in the face of these various approaches? Fortunately, perfusion pressure support of varying degrees is a theme that is common to of all of them. The characteristic behavior of CBF after head injury is an initially low CBF followed by a gradual increase over a period of 48 to 72 hours to normal or sometimes even slightly hyperemic levels. [42] [274] [287] Accordingly, aggressive support of CPP in the 60- to 70-mm Hg range during that period will be the most reasonable general approach. Note that in patients with subarachnoid blood, a second period of low CBF may occur from days 4 to 10 after injury, apparently on a vasospasm-related basis.[40] [288] [289] It should also be understood that there are exceptions to the foregoing generalizations about CBF after TBI. Although an initially low postinjury CBF is probably the most common clinical occurrence, hyperemia does occur.[287] [288] [290] It tends to develop in patients with mass lesions rather than contusions, although even these patients have an immediate postinjury period of low CBF, with delayed hyperemia peaking at 24 hours or later.[39] [40] [270] [287] [290] Hyperemia may also be common in children.[291] Nonetheless, in the absence of measures of CBF or brain tissue well-being (both of which are uncommonly available), careful maintenance of a CPP of 60 to 70 mm Hg in the first 72 hours after TBI will be appropriate and is common practice in a head-injured adult.[139] [271] [272] [274] [279] [292] [293] A CPP target of 45 mm Hg has been recommended for children.[294] With these firm recommendations offered, it must be acknowledged that convictions will vary and anesthesiologists should come to an understanding with the local traumatologists and neurosurgeons regarding blood pressure targets.
In the ideal situation, management of CPP is "targeted" to the pathophysiology that prevails in the individual patient.[295] However, techniques to discriminate the various flow states (CBF measurement, transcranial Doppler, SjVO2 , brain tissue PO2 , see later) are not universally available or applied (nor yet proven to lead to improvement in outcome).
The use of hypocapnia has been reviewed in detail in the section "Management of PaCO2 ." Hyperventilation has long been a standard component of the management of TBI patients perceived to be at risk for increased ICP. However, evidence is increasing that hyperventilation is potentially deleterious[26] [27] [28] [30] [32] [34] [35] [36] and should not be overused. That evidence suggests that hyperventilation and the concomitant vasoconstriction can result in ischemia,[25] [35] [36] [37] especially when baseline CBF is low,[37] as is likely to be the case in the first 48 to 72 hours after head injury.[28] [38] The expert panel mentioned earlier, convened by the Brain Trauma Foundation, specified that "chronic prophylactic hyperventilation should be avoided during the first 5 days after severe TBI and particularly during the first 24 h."[296] The available information argues that hyperventilation should be used selectively rather than routinely in the management of TBI patients. Maintaining ICP at less than 20 mm Hg, preventing or reversing herniation, minimizing retractor pressure, and facilitating surgical access are still important objectives in the management of TBI patients, and to the extent that hyperventilation contributes to these objectives it is still appropriate. Once again, the anesthesiologist should agree on management parameters with the surgical team at the outset of a procedure.
Fluid management of a head-injured patient was addressed in the section "Intravenous Fluid Management." The important principles are that fluids should invariably be chosen to prevent a reduction in serum osmolarity and should probably be chosen to prevent a profound reduction in colloid oncotic pressure; specifically, in the circumstances of large-volume resuscitation (arbitrarily, greater than half a circulating volume), a mix of colloids and crystalloids is probably appropriate. The clinical objective should be maintenance of intravascular normovolemia, in part as an adjunct to MAP and CPP support. A chronic negative fluid balance, as can occur with the combination of modest fluid restriction and liberal use of osmotic diuretics, has been shown to be deleterious and should be avoided.[139] Remember also that a severely injured brain can liberate sufficient thromboplastin into the circulation to result in consumptive coagulopathy. Appropriate laboratory tests and replacement should be performed.[297] The clinician may also find that determination of serum osmolarity early in the course of anesthetic management is useful in appreciating the cumulative effects of previous administration of mannitol. The use of hypertonic solutions and the relevant attributes of colloid solutions were also discussed in the section "Intravenous Fluid Management."
Numerous centers have studied or made use of SjVO2 monitoring as a guide to the clinical management of head-injured patients.[31] [35] [36] [37] [43] [44] [266] [272] [298] [299] The underlying concept is that marginal or inadequate CBF will result in increasing oxygen extraction, a widening arteriovenous content difference, and decreasing jugular venous PVO2 or SjVO2 . There have been numerous reports of improvement in SjVO2 as a consequence of reducing hyperventilation, increasing MAP, or inducing hypervolemia. [300] The availability of intravascular catheters that permit continuous monitoring of SjVO2 has made the technique more practical.[301] SjVO2 measurement makes an assessment of global oxygen extraction. Accordingly, it might be expected to have limited sensitivity in highly
Some technical limitations are inherent in SjVO2 monitoring.[302] Catheter placement must be very precise to avoid contamination by noncerebral venous blood or attenuation of light return (with optical catheters) because of vessel wall abutment. Even in experienced hands, the false-positive rate can be significant.[37] An additional limitation inherent in unilateral placement of the catheter is the observation by Stocchetti and coworkers that there was an average side-to-side difference between simultaneous jugular bulb saturations of 5.3% ± 5% and that side-to-side differences in hemoglobin saturation of up to 15% were common. [303] These and other authors have questioned the reliability of data obtained from a unilateral SjVO2 catheter.[303] [304] The next clinical problem is the matter of what constitutes an abnormal value. Normal subjects at rest may have SjVO2 values between 50% and 75%. A currently used definition of abnormal is less than 50% for 5 minutes.[305] However, there has been limited opportunity to correlate SjVO2 threshold values with outcome.
In spite of the reported success with SjVO2 monitoring,[44] [300] [306] we do not believe that the method is sufficiently well defined to justify advocating widespread intraoperative application. Further experience and clinical investigation are required to define the correct role and mode of application of SjVO2 monitoring. At present, it appears potentially useful as a trend monitor that may serve to identify the level of CPP below which cerebral perfusion begins to be compromised. However, the SjVO2 level at which that compromise is critical has not been identified. The technique also has a potential use beyond identifying patients with low SjVO2 and inadequate CBF. High SjVO2 values may serve to identify a patient with elevated ICP in whom hyperemia is an important contributing factor and in whom aggressive attempts to decrease CBF (e.g., hyperventilation, barbiturates) may be beneficial.
Small-diameter intraparenchymal electrodes are available that allow measurement of brain tissue PtiO2 (and sometimes pH and PtiCO2 ). They have been used to monitor cerebral well-being during the ICU management of both TBI and SAH patients and to assess the effect of operative interventions.[228] [243] [274] [307] [308] [309] A PtiO2 of 20 mm Hg is viewed as normal, and values less than 10 mm Hg are assumed to convey a risk of hypoxic injury. They suffer from the inverse of the problem that prevails with SjVO2 monitoring in that they are very focal monitors that assess the oxygenation status of only small regions of brain surrounding the tip. If they are placed remote from focal injuries in a traumatized brain, they may not "see" adverse events in salvageable perilesional tissue. They may similarly fail to be a useful therapeutic guide if they are within irredeemably injured brain. Thus far, their use is neither standardized nor widespread.
Mild induced hypothermia has already crept into the management of neurosurgical procedures in which there is a perceived risk of ischemic injury. To date, these procedures have encompassed principally aneurysm surgery, although proof of a favorable effect on outcome in this context has not yet been established (see the earlier section "Hypothermia"). However, its efficacy in reducing damage when induced after experimental head injury has also been demonstrated,[156] and its relevance to the management of a head injury patient has been the subject of small, prospective controlled trials in at least four centers.[158] [159] [160] [161] Because these single-center trials appeared to indicate good patient tolerance of sustained mild hypothermia (32°C to 34°C), as well as improvement in ICP, cerebral oxygen supply/demand, and outcome, a multicenter trial was performed. That trial revealed no overall benefit of hypothermia.[162] However, post hoc analysis demonstrated that patients who were hypothermic (<35°C) on admission to the hospital benefited from hypothermia whereas those who were normothermic on admission did not. Patients older than 45 years derived no benefit at any admission temperature. As a result, hypothermia has no established role in management of head injury as of this writing. However, a follow-up trial is under way. Patients 16 to 45 years of age with GCS scores less than 8 who arrive at the hospital with a bladder temperature less than 35°C are to be randomized to cooling to 32.5°C to 33.5°C (within 4 hours of injury) for 48 hours or passive rewarming to normothermia. Outcome will be assessed at 6 months.
In ideal circumstances, neurosurgical consultation will be readily available, and appropriately, the anesthesiologist will rarely have to make this decision. It may, however, be necessary for the anesthesiologist to participate in this decision. Relevant variables include the following:
|
|
|
|
|
|
|
|
|
|
|
|
|