|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contemporary management of intracranial aneurysms calls for early surgical intervention after SAH. The definition of "early" varies up to and including the first 72 hours after bleeding.[174] This approach was originally applied only to patients in the better neurologic grades, that is, grades I to III and perhaps IV of the World Federation of Neurosurgeons (WFNS) classification ( Table 53-8 ) or grades I to III of the Hunt-Hess classification ( Table 53-9 ), but more recently it has been extended to patients with higher grades. [175] If early intervention is not feasible, surgery is usually delayed for at least 2 weeks to be safely beyond the period of maximal risk of vasospasm (i.e., days 4 to 12 after SAH). Currently, some clinicians are advocates of the so-called ultra-early intervention, which entails clipping within 18 hours of the initial SAH.[176]
| WFNS Grade | GCS Score | Motor Deficit |
|---|---|---|
| I | 15 | Absent |
| II | 14–13 | Absent |
| III | 14–13 | Present |
| IV | 12–7 | Present or absent |
| V | 6–3 | Present or absent |
| GCS, Glasgow Coma Scale; SAH, subarachnoid hemorrhage. | ||
| From Drake CG, Hunt WE, Sano K, et al: Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg 68:985, 1988. | ||
| Category | Criteria * |
|---|---|
| Grade I | Asymptomatic or minimal headache and slight nuchal rigidity |
| Grade II | Moderate to severe headache, nuchal rigidity, no deficit other than cranial nerve palsy |
| Grade III | Drowsiness, confusion, or mild focal deficit |
| Grade IV | Stupor, moderate to severe hemiparesis, possibly early decerebrate rigidity and vegetative disturbances |
| Grade V | Deep coma, decerebrate rigidity, moribund appearance |
| From Hunt WE, Hess RM: Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14, 1968. | |
The rationale for early clipping is several-fold. The sooner the aneurysm is clipped, the less the likelihood of rebleeding (and rebleeding is the principal cause of death in patients hospitalized after SAH[177] ). Second, management of the ischemia caused by vasospasm involves volume loading and induced hypertension. Early clipping of the aneurysm eliminates the risk of rebleeding associated with this therapy. Vasospasm appears to be related to the presence of blood in the basal cisterns in the vicinity of the circle of Willis. Some of this blood can be removed at the time of aneurysm clipping, and accordingly, early clipping not only makes therapy for vasospasm safer but may also reduce the incidence and severity of the problem. In addition, early access to the circle of Willis allows direct instillation of tissue plasminogen activator into the basal cisterns to further aid in clearing clot from the circle of Willis.[178] [179] The results of the initial evaluations of tissue plasminogen activator therapy are suggestive of a substantial benefit, although this therapy remains experimental. Previous surgical practices entailed maintaining the patient at bed rest until approximately day 14, when the period of risk for spasm had passed. Early aneurysm clipping reduces the period of hospitalization and decreases the incidence of medical complications (deep venous thrombosis, atelectasis, pneumonia) associated with a lengthy period of enforced bed rest.
Early intervention can make the intraoperative course more difficult. Brain tissue in the early post-SAH period is likely to be more edematous than after a 2-week delay. A mild degree of hydrocephalus is common after blood contaminates the subarachnoid space.[180] In fact, 10% to 20% of SAH victims may actually require CSF shunting at some point in their course.[181] [182] Early intervention may also enhance the risk of intraoperative aneurysm rupture somewhat because of the lesser time for clot to organize over the site of the initial bleeding. All of these issues place a substantial premium on techniques designed to reduce the volume of the intracranial contents (see the section "Control of Intracranial Pressure/Brain Relaxation") to facilitate exposure and minimize retraction pressure.
Many patients scheduled for intracranial aneurysm clipping will come directly from the ICU, and elements of their management there may influence their immediate preoperative status.
In some patients, the syndrome of inappropriate antidiuretic hormone secretion (SIADH) develops after SAH, and it will be appropriately managed with fluid restriction. However, hyponatremia after SAH is more likely to be a consequence of the cerebral salt-wasting syndrome that probably occurs as a result of the release of a natriuretic peptide (similar to what occurs in the heart).[183] [184] [185] [186] [187] [188] [189] Cerebral salt-wasting syndrome is characterized by the triad of hyponatremia, volume contraction, and high urine sodium concentrations (>50 mmol/L). Distinction between this syndrome and SIADH is important. SIADH, which is characterized by normovolemia or mild hypervolemia, is treated by volume restriction. Cerebral salt wasting is associated with a contracted intravascular volume. [186] [190] [191] Fluid restriction and further volume contraction may be especially deleterious in a post-SAH patient[183] [192] and should be avoided. Although clinical distinction between these two causes of hyponatremia (SIADH and cerebral salt wasting) may be difficult, management of both is relatively simple: administration of isotonic fluids with intravascular normovolemia used as the end point.
When neurologic deterioration occurs subsequent to the patient's initial period of stabilization, vasospasm is frequently the cause. Drowsiness is a common initial clinical sign. In patients in whom there is clinical suspicion or angiographic demonstration of vasospasm, surgery is commonly deferred. If it is to proceed, CPP should be maintained intraoperatively in a high-normal range. This recommendation is contrary to the time-honored patterns of intraoperative management, which have characteristically emphasized hypotension. However, neurosurgeons recognize the potential for induced hypotension to cause or aggravate cerebral ischemia in patients with vasospasm.[193] [194] This concern extends even to a WFNS grade I patient who may have regions of cerebral ischemia[45] that are subclinical when the patient is normotensive. Vasospasm is thought to be caused by the breakdown products of hemoglobin from blood that has accumulates around vessels of the circle of Willis after SAH. A specific mechanism/mediator has not been identified, but the focus of the most current research is on endothelin as a potential culprit.[195] [196]
In the ICU, the regimens used to treat vasospasm generally involve some combination of hypervolemia, hemodilution, and hypertension. The science behind hypervolemic-hypertensive therapy is "soft," and the efficacy of "triple H" therapy has not been proved by prospective study.[197] The relative importance of the rheologic and pressure effects is undefined, although there is evidence for the relevance of blood pressure elevation in isolation.[198] [199] Phenylephrine and dopamine are the most commonly
Calcium channel blockers are now an established part of the management of SAH. Administration of nimodipine has been shown to decrease the incidence of morbidity from cerebral ischemia after SAH.[200] [201] [202] However, these studies have failed to demonstrate any reduction in the incidence of vasospasm as detected by angiography,[201] which suggests that the beneficial effect of these agents may be the result of effects on neurons rather than vascular smooth muscle. Patients coming to the operating room after SAH should be receiving nimodipine. The available information suggests that the use of nimodipine causes very little hemodynamic disturbance.[203] Nimodipine must be administered orally, and nicardipine has been evaluated as an intravenous alternative. The multicenter nicardipine trial[204] [205] revealed a reduced incidence of symptomatic vasospasm but no improvement in outcome. As a consequence, nimodipine is used more commonly.
Antifibrinolytics were administered in the past in an attempt to reduce the incidence of rebleeding. Even though they accomplish the latter, overall morbidity is not improved as a result of an increase in the incidence of ischemic symptoms and hydrocephalus.[206] [207] [208] However, many of the observations that have discouraged the use of antifibrinolytics were made in the context of prolonged administration before delayed surgery. Recent results of brief administration of tranexamic acid before early surgery suggested a decreased rate of rebleeding without any apparent increase in ischemic complications, and it is possible that the role of antifibrinolytics will be reconsidered.[209]
ECG abnormalities can occur in patients who have sustained SAH. In addition to the classic "canyon T waves" ( Fig. 53-14 ), nonspecific T-wave changes, QT prolongation, ST-segment depression,
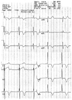 Figure 53-14
Electrocardiographic abnormalities associated with subarachnoid
hemorrhage (SAH). The "canyon" T waves that may be seen after SAH are evident.
Figure 53-14
Electrocardiographic abnormalities associated with subarachnoid
hemorrhage (SAH). The "canyon" T waves that may be seen after SAH are evident.
Important considerations include the following:
An arterial line is invariably appropriate. A CVP line may be relevant if institutional practices involve large doses of mannitol to promote brain relaxation, and it may be used in older patients to guide volume replacement in the event of bleeding.
Any technique that permits proper control of MAP is acceptable. However, in the face of increased ICP or a tight surgical field, an inhaled anesthetic technique may be less suitable. Prevention of paroxysmal hypertension is the only absolute requirement in patients undergoing aneurysm clipping. Rebleeding kills, [177] and the poorly organized clot over the aneurysms of patients undergoing early post-SAH clipping makes them particularly prone to rebleeding. Rebleeding at induction is a frequently fatal event. The escaping arterial blood is more likely to penetrate brain substance because it cannot dissect through the CSF space (filled with clot), and the increase in ICP is extreme because of poor compliance of the intracranial space (swollen brain, hydrocephalus).
The routine use of induced hypotension is diminishing (see the earlier section "Management of Blood Pressure").[193] [194] Nonetheless, the anesthesiologist should be prepared to lower blood pressure immediately and precisely if called on to do so. Preparation must occur before the episode of bleeding. We prepare a sodium nitroprusside infusion before induction. It is placed in line at a Y-injection port at the hub of the CVP or intravenous catheter. The carrier drip should flow steadily so that any change in the nitroprusside infusion rate will be reflected as rapidly as possible in the central compartment. There are theoretical pros and cons for various hypotensive drugs. Data indicate that regimens entailing the use of a drug that is also a cerebral vasodilator (isoflurane, nitroprusside) are preferable in terms of brain oxygen delivery to approaches that do not involve cerebral vasodilation (trimethaphan, controlled hypovolemia).[220] Deep isoflurane and nitroprusside are the most commonly used regimens, although other drugs may be suitable. The choice should ultimately be made on the basis of which regimen, in the hands of the individual practitioner, results in the most precise control of MAP. On occasion, the anesthesiologist will be asked to control MAP in the range of 30 to 50 mm Hg in the face of the active arterial bleeding. This task can be extremely difficult in a patient who is hypovolemic at the beginning of the bleeding episode. Accordingly, it is our practice to maintain normovolemia.
Hypertension may be requested during periods of temporary arterial occlusion (see the later section "Trapping") to augment collateral CBF.[152] In addition, after clipping of the aneurysm, some surgeons will puncture the dome of the aneurysm to confirm adequate clip placement and may request transient elevation of systolic pressure to 150 mm Hg. Phenylephrine is suitable in either instance.
Hypocapnia has traditionally been used as an adjunct to brain relaxation. This practice has also been questioned on the basis of concern that it will aggravate ischemia (see the earlier section "Management of PaCO2 "). The ICP/brain relaxation circumstances should probably dictate its use or avoidance.
Some surgeons use elective drainage to facilitate exposure. It is extremely effective, effective to the point that the energetic application of other brain volume-reducing techniques is almost unnecessary. It is appropriate, when placing a lumbar CSF drain, to avoid excessive loss of CSF. A sudden reduction in the transmural pressure gradient across the dome of the aneurysm (by a sudden reduction in ICP after substantial CSF drainage) should be avoided lest such decompression encourage rebleeding.[221] After having verified the patency of the lumbar drainage
Some surgeons make relatively aggressive use of mannitol (e.g., 2 g/kg). In part, it is used to shrink the brain and thereby facilitate exposure and reduce retractor pressure. There is evidence that it may have additional benefits. Specifically, data derived from both animals and humans indicate that mannitol may have a CBF-enhancing effect in regions of moderate CBF reduction.[222] [223] [224] [225] The mechanism is not defined. However, a reduction in interstitial tissue pressure around capillaries or an alteration in blood rheology (or both) has been proposed to contribute. Typically, mannitol is administered in a dose of 1 mg/kg just before dural opening. Surgeons who believe in the flow-enhancing effect may request a second 1 mg/kg approximately 15 minutes before an anticipated temporary occlusion.
It is occasionally necessary for the surgeon to "trap" the aneurysm (i.e., to temporarily occlude the vessel on either side of the aneurysm) to complete dissection of the neck and apply the clip. This technique is more common with larger aneurysms. With giant aneurysms in the vicinity of the carotid siphon, the inferior occlusion may be performed at the level of the internal carotid artery through a separate incision in the neck. A clinical survey by Samson and colleagues of the neurologic outcome after temporary occlusion in normothermic, normotensive adults revealed that occlusion times less than 14 minutes were invariably tolerated. The likelihood of an ischemic injury increased with longer occlusion and reached 100% with occlusion in excess of 31 minutes.[226] In another institution, the threshold for ischemic injury was 20 minutes of occlusion. [152] Typically, it will be appropriate to support MAP at high-normal levels during periods of occlusion to facilitate collateral CBF.
Maintenance of MAP to ensure collateral flow and perfusion under retractors, efficient brain relaxation to facilitate surgical access and reduce retractor pressure, limitation of the duration of episodes of temporary occlusion, and perhaps mild hypothermia (see the section "Hypothermia") are the important brain protection techniques. Specific anesthetics have also been used to protect the brain (see discussion in Chapter 21 ). Etomidate and propofol are au courant. However, there have been no convincing laboratory demonstrations that propofol provides any greater tolerance to a standardized ischemic insult than anesthesia with a volatile anesthetic does. Attempts to demonstrate protection by etomidate in a animal model of focal ischemia actually demonstrated an adverse effect of etomidate.[227] Furthermore, a clinical investigation during aneurysm clipping revealed decreases in brain tissue PO2 in association with the administration of etomidate, which contrasted with the increases in brain PO2 that occurred with the introduction of desflurane. During subsequent temporary vessel occlusion, tissue pH decreased alarmingly in patients receiving etomidate and was unchanged with desflurane.[228] Although the available data collectively do not support an assertion that etomidate is harmful in the setting of aneurysm surgery, we think that they should dissuade any anesthesiologist from advocating the administration of etomidate as a protective substance in the context of aneurysm surgery.[229] [230]
With respect to the volatile anesthetics, attempts in the laboratory to confirm the once-proclaimed protective efficacy of isoflurane have not demonstrated any differences among the various volatile anesthetics in terms of their influence on outcome after focal or global ischemia.[227] [231] [232] [233] Nor has there been any demonstration of greater protective efficacy with concentrations of volatile anesthetics sufficient to cause EEG suppression as opposed to more modest levels (e.g., 1.0 MAC).[233] [234] Nonetheless, these animal investigations do suggest that a standardized experimental ischemic insult is better tolerated, relative to the awake state, by animals receiving a volatile anesthetic.[232] [233] [235] In addition, data derived in animals also suggest that there may be a relative protective advantage to an anesthetic technique that includes a volatile anesthetic versus a strict nitrous oxide-narcotic technique. [233] [235] However, the magnitude of the differences among anesthetics and the absence of proof of relevance in patients preclude advocacy of a particular anesthetic regimen in a standard text. The important anesthetic objectives are precise hemodynamic control and timely wake-up, and these two constraints should dictate the choice of the anesthetic regimen for most aneurysm procedures. Among anesthetics, it is only the barbiturates for which additional protective efficacy has been demonstrated convincingly (see Chapter 21 ). Because of their potentially adverse effects on hemodynamics and wake-up, they are not ideal for routine administration. Barbiturates should probably be reserved for situations in which prolonged vessel occlusion is unavoidable, and in that circumstance it would be ideal if the ischemic hazard were first confirmed by observation of the EEG response to temporary occlusion.[236]
As noted in the earlier section "Hypothermia," the efficacy of mild hypothermia in the context of aneurysm surgery has not been confirmed in humans, although recruitment of patients to a multicenter, prospective comparison of normothermia and hypothermia has been completed and the results are awaited. Nonetheless, neurosurgical teams in many institutions are lowering body temperature to the range of 32°C to 34°C for procedures in which vessel occlusion may occur. The institutions that use lower temperatures are those in which the team is willing to accept a delay in emergence from anesthesia. That delay results from the necessity to maintain anesthesia long enough to achieve sufficient rewarming to avoid the extreme hypertension that can occur when a patient is awakened at low body temperature.
Both evoked responses and the EEG have been used,[236] [237] though neither widely. EEG monitoring can be used as a guide to management during the period of flow interruption or to guide the administration of CMR-reducing anesthetics given before occlusion.[236] At some institutions, the surgeon places an electrode strip over the region of cortex at risk during the intended occlusion. However, the more commonly used skin surface frontal-mastoid derivation is probably sufficient to reveal a major ischemic event. The EEG can be either the conventional "raw" polygraph or a processed derivative. In most circumstances, if occlusion is deemed necessary, a temporary occlusion is performed and the EEG observed. If the EEG shows significant slowing, the usual practice is to reposition the clip or elevate MAP by some combination of phenylephrine and lightening of anesthesia (or both) to find a way to carry out temporary clipping without a major EEG disturbance. If this goal cannot be accomplished and a sustained period of occlusion seems likely, it may be appropriate to administer barbiturates (see earlier) to produce burst suppression.
Intraoperative angiography is becoming an increasingly common component of the management of intracranial aneurysms. It does not have substantial implications for the anesthesiologist. However, apparatus around the patient's head must be organized to allow C-arm access without snagging of airway and monitoring equipment. In addition, the radiologist must have access to the groin vessels. The vascular access sheath, for a patient who will ultimately be in the lateral position, is easiest to use when the nondependent femoral artery is chosen. The rate of administration of heparin "flush" through the vascular access sheath should be monitored.
The most common procedures are performed for aneurysms arising in or close to the circle of Willis. The vessel of origin may be the anterior communicating artery, the middle cerebral artery, the anterior cerebral artery, the ophthalmic artery, the tip of the basilar artery, the posterior communicating artery, and less frequently, the posterior cerebral artery. These procedures will all be relatively similar for the anesthesiologist and will typically require a supine position with the head turned slightly away from the operative side.
Access to the origin of the ophthalmic artery, which is the first intradural branch of the carotid artery, is made difficult by the anterior clinoid process and the optic nerve. As a result, these aneurysms frequently require temporary vascular occlusion. The surgeon will commonly first expose the carotid artery in the neck. When the stage of seeking definitive access to the neck of the aneurysm is reached, the surgeon will occlude the carotid artery in the neck first and then the intracranial portion of the carotid artery immediately proximal to the origin of the posterior communicating artery. The surgeon may also cannulate the isolated segment and place it to suction, which will entail modest ongoing blood loss as a consequence of retrograde flow from meningeal and hypophyseal vessels.
These procedures are typically performed in the lateral position. The exposure may involve a combined middle and posterior fossa approach, with some attendant, though minor risk of VAE. Cortical or skin surface EEG monitoring is of less relevance with vertebral-basilar aneurysms. Auditory or somatosensory evoked responses, or both, have been used.[238] As in any other procedure involving the potential for mechanical or vascular injury to the brainstem, cardiovascular responses should be monitored, and sudden changes in response to surgical manipulation should prompt immediate notification of the surgeon. Spontaneous ventilation has also been shown to have an important role to play during surgical manipulation of the vertebral arteries, the vertebrobasilar junction, and the middle portion of the basilar artery. Apnea, gasping, or other sudden changes in ventilatory pattern during manipulation of the vasculature provide important, though somewhat nonspecific warning of compromise of the vascular supply of the brainstem. [239] [240] Management of these aneurysms will occasionally require cardiopulmonary bypass and deep hypothermic circulatory arrest.
These "aneurysms" are more appropriately managed according to considerations relevant to AVMs. Such considerations include anticipation of the possibility of the cerebral dysautoregulation phenomenon and are considered later.
For most intracranial AVMs, the general considerations are similar to those appropriate to aneurysm surgery: avoidance of acute hypertension and the capability of accurately manipulating blood pressure in the event of bleeding. A problem that is specific to AVMs is the phenomenon known as "perfusion pressure breakthrough," or cerebral dysautoregulation.[241] [242] It is characterized by an often sudden engorgement and swelling of the brain, sometimes with a relentless cauliflower-like protrusion from the cranium. It tends to occur in the advanced stages of lengthy procedures on large AVMs. The phenomenon is not entirely understood. However, it has been attributed to acute obliteration of the high-volume, low-resistance pathway (the AVM) that for many years has been stealing blood from the surrounding tissue. The result is abrupt diversion of the AVM's flow to the vasculature in adjacent and previously marginally perfused brain tissue.[243] It has been theorized that these tissues have long been maximally vasodilated without the need to ever vasoconstrict and have become incapable of doing so. However, not all of the available information is entirely consistent with this mechanism,[242] [244] [245] and some evidence indicates that the neovascularization occurring in response to
The management constraints are essentially the same as those relevant to aneurysm surgery. Institutional practices will vary. We do not use induced hypotension unless required to by bleeding. We reason that the effects of devascularizing the AVM on the surrounding brain will be best appreciated if the devascularization occurs at normal pressure. If refractory brain swelling occurs, we can then also lower MAP as part of attempts to control the swelling. The rationale is that blood flow through the involved area is pressure passive and will decrease as MAP is reduced. With severe episodes of swelling, we have used (in addition to hypotension, which we use cautiously because of the associated risk of ischemia) hypocapnia, hypothermia, and barbiturates. The latter three techniques probably serve to reduce the bulk of only normal brain tissue—hypocapnia through a direct effect on CBF and barbiturates and hypothermia through the coupled effects of reduction of CMR on CBF. Induced hypothermia is also an adjunct to minimizing barbiturate doses. In all of neurosurgery we seek to prevent postoperative hypertension. However, it is in AVM surgery that this objective should be accomplished with greatest care because of the concern that edema or hemorrhage will develop in the "dysautoregulating" brain adjacent to the resected AVM if hypertension occurs.
|
|
|
|
|
|
|
|
|
|
|
|
|