|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There are a certain subset of neonates, infants, and children with congenital heart disease who require extensive repair of complex congenital heart defects using DHCA. This technique facilitates precise surgical repair under optimal conditions, free of blood or cannulas in the operative field, providing maximal organ protection, and often resulting in shortened total CPB time. The scientific rationale for the use of deep hypothermic temperatures rests primarily on a temperature-mediated reduction of metabolism. Whole body and cerebral oxygen consumption during induced hypothermia decreases the metabolic rate for oxygen by a factor of 2 to 2.5 for every 10°C reduction in temperature.[104] These results are consistent with in vitro models, which relate temperature reduction to a decrease in the rate constant of chemical reactions, as originally described by Arrhenius using the equation k = Ae−RT . The reduction in oxygen supply during deep hypothermic low-flow CPB is associated with preferential increases in vital organ perfusion (e.g., to the brain) and increased extraction of oxygen.[105] Therefore, to some extent, deep hypothermic low-flow CPB exerts a protective effect by reducing the metabolic rate for oxygen, promoting preferential organ perfusion, and increasing tissue oxygen extraction.
The duration of the safe period of DHCA has not clearly been delineated. [98] Information from a number of studies shows that an arrest period up to at least 40 minutes is tolerated without long-term sequelae. Beyond this duration, the incidence of permanent and transient neurologic sequelae may increase. Both the duration of the arrest period and variations in perfusion technique during cooling and rewarming may influence the development of these problems. The effect of deep hypothermia on tissue metabolism and oxygen consumption and extraction clearly does not explain the entire protective effect of "safe" DHCA, however. Cortical PO2 and PCO2 levels indicate basal cerebral metabolic activity during DHCA (i.e., anaerobic metabolism). During brain ischemia, excitatory amino acids such as glutamate and aspartate are released and are putative mediators of ischemic damage.[49] [100] [101] [103] [106] [107] Hypothermia has been shown to significantly decrease the release of excitatory amino acids, suggesting another mechanism besides metabolism reduction for its protective effect.[108] In addition, membrane changes that transform a normal semiliquid to a semisolid form during hypothermia may act to prevent calcium influx during reperfusion and thereby account for additional protection noted in some experimental models.[109]
Although all organ systems are at risk for the development of ischemic and reperfusion injury, as manifested by lactate and pyruvate production during DHCA, the brain appears to be the most sensitive to and the least tolerant of these effects. Brainstem and cortical evoked potentials as well as processed electroencephalograms are altered after DHCA.[110] [111] [112] The abnormalities in the evoked potentials appear to be related to the duration of DHCA and are attributed to altered metabolism. During reperfusion after the arrest period, CBF and metabolism remain depressed in neonates and small infants ( Fig. 51-9 ; also see Fig. 51-6 ). [56] Importantly, during the use of these extremes of temperature, it appears that autoregulation is lost and cerebral perfusion becomes highly dependent on the conduct of extracorporeal perfusion and presumably post-bypass hemodynamic performance.
Current controversy exists regarding the immediate-term and long-term neuropsychologic effects of DHCA. Early reports regarding the long-term consequences of DHCA on brain development and intelligence were conflicting.[113] Transient neurologic dysfunction and other reversible cerebral injuries have been reported. These transient, subtle neuropsychologic disturbances have led investigators to examine more systematically the long-term outcome after DHCA.
A number of more sophisticated studies examining the outcome after DHCA have been performed. In a randomized clinical trial comparing the incidence of brain injury following DHCA or low-flow CPB, DHCA was demonstrated to have longer electroencephalographic recovery times and a higher incidence of clinical seizures in the early postoperative period.[114] Patients in the DHCA group also had a higher incidence of neurologic abnormalities and poor motor function at 1 year of age and poor expressive language and motor development at 2½ years, particularly those who exhibited early postoperative seizures. [115] Recent reports from the same clinical trial show that patients in the DHCA cohort continued to have worse motor coordination and planning and speech abnormalities at 4 years of age.[116] [117] [118] Of interest, both the DHCA and the low-flow CPB groups had lower cognitive and motor performance compared to the general population. This finding suggests that factors other than DHCA
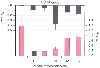 Figure 51-9
Bar graph of variations in cytochrome oxidase (cyt a,a3
)
near-infrared spectroscopic signals and cerebral metabolic rate for oxygen (CMRO2
)
in subjects with deep hypothermic circulatory arrest (DHCA). Each point of cyt a,a3
represents mean ± SE in six subjects; CMRO2
values are mean ±
SD. Negative values in cyt a,a3
represent relative decreases in quantity
of oxidized enzyme. *, CMRO2
and cyt a,a3
are significantly
different from control, p < .05.
Figure 51-9
Bar graph of variations in cytochrome oxidase (cyt a,a3
)
near-infrared spectroscopic signals and cerebral metabolic rate for oxygen (CMRO2
)
in subjects with deep hypothermic circulatory arrest (DHCA). Each point of cyt a,a3
represents mean ± SE in six subjects; CMRO2
values are mean ±
SD. Negative values in cyt a,a3
represent relative decreases in quantity
of oxidized enzyme. *, CMRO2
and cyt a,a3
are significantly
different from control, p < .05.
One clinical study has suggested that a pH-stat blood gas management strategy during CPB is associated with an improved neuropsychologic outcome in children. [117] This was a retrospective developmental study with a core of patients who had undergone surgery for transposition of the great arteries. The authors found a strong positive correlation between arterial PCO2 during cooling before circulatory arrest and developmental score. This suggested that children undergoing α-stat blood gas management strategy had a worse developmental outcome than those in whom a pH-stat strategy was employed. In a randomized clinical trial of neonates undergoing cardiac surgery using deep hypothermic circulatory arrest, pH-stat management was noted to have faster electroencephalographic recovery times and fewer postoperative seizures compared with an α-stat bypass group.[119] [120] The beneficial effect of pH-stat management clinically remains preliminary.
Certain experimental studies have also suggested the superiority of pH-stat strategy ( Fig. 51-10 ). In one study, pH-stat animals had increased CBF during cooling and better recovery of cerebral adenosine triphosphate (ATP) and intracellular pH after arrest and reperfusion. [122] Brain water content was also less in the pH-stat group. These studies suggested that pH-stat CPB may have protective mechanisms due to an increased rate of brain cooling. In more recent experimental studies comparing pH-stat and α-stat CPB on cerebral oxygenation, the cerebral protective effect of pH-stat management was demonstrated and indicated that the kinetics of cerebral deoxygenation may contribute to the mechanism of protection ( Fig. 51-11 ).[123] [124] [125] Clearly, more work needs to be done in this area before pH-stat can be definitively recommended.[126] However, preliminary experimental and clinical studies suggest a superiority of this technique in certain patient groups, especially those with aortopulmonary collateral circulation.
Because of the potential for neurologic dysfunction after DHCA, some institutions use low-flow deep hypothermic CPB as an alternative technique. [26] Because low-flow bypass can produce ischemia if flow is too low
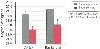 Figure 51-10
Cortical and basilar brain temperature at the end of
circulatory arrest (90 minutes) in animals with systemic-pulmonary shunts. Note
the significantly lower brain temperatures in the pH-stat animals. (From
Kirshbom PM, Skaryak LA, DiBernardo LR, et al: Effect of aortopulmonary collaterals
on cerebral cooling and metabolic recovery during cardiopulmonary bypass and circulatory
arrest. Circulation 92(9 Suppl):II-490, 1995.)
Figure 51-10
Cortical and basilar brain temperature at the end of
circulatory arrest (90 minutes) in animals with systemic-pulmonary shunts. Note
the significantly lower brain temperatures in the pH-stat animals. (From
Kirshbom PM, Skaryak LA, DiBernardo LR, et al: Effect of aortopulmonary collaterals
on cerebral cooling and metabolic recovery during cardiopulmonary bypass and circulatory
arrest. Circulation 92(9 Suppl):II-490, 1995.)
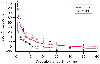 Figure 51-11
Cortical oxygen saturation (SCO2
)
during deep hypothermic circulatory arrest in the pH-stat and the α-stat groups.
The SCO2
half-life during arrest was
significantly greater in the pH-stat than in the α-stat group. (From
Kurth CD, O'Rourke MM, O'Hara IB: Comparison of pH-stat and alpha-stat cardiopulmonary
bypass on cerebral oxygenation and blood flow in relation to hypothermic circulatory
arrest in piglets. Anesthesiology 89:110, 1998.)
Figure 51-11
Cortical oxygen saturation (SCO2
)
during deep hypothermic circulatory arrest in the pH-stat and the α-stat groups.
The SCO2
half-life during arrest was
significantly greater in the pH-stat than in the α-stat group. (From
Kurth CD, O'Rourke MM, O'Hara IB: Comparison of pH-stat and alpha-stat cardiopulmonary
bypass on cerebral oxygenation and blood flow in relation to hypothermic circulatory
arrest in piglets. Anesthesiology 89:110, 1998.)
Other factors such as surface cooling, anesthetic agents, and cerebral protective agents may influence and modify the effects of deep hypothermic low-flow CPB and DHCA. The potential use of certain pharmacologic agents such as anesthetic drugs, barbiturates, lidocaine, and calcium channel blockers is unknown. There are no clinical studies in children systematically examining the influence of these pharmacologic agents on cerebrovascular physiology or neurologic outcome. Therefore, the use of these agents remains entirely speculative. Clearly, further study of the long-term effects of DHCA on neuropsychologic outcome in children is necessary. Fundamental questions regarding deep hypothermic cardiopulmonary bypass (DHCPB) with low flow versus DHCA also need to be addressed further. Equally important, the manner in which the patient is cooled and rewarmed may affect outcome[58] [59] and merits further investigation, even before the testing of pharmacologic therapies.
|
|
|
|
|
|
|
|
|
|
|
|
|