|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although many of the principles governing modern pediatric cardiovascular anesthesia are similar to those that guide anesthetic management of the adult cardiac patient, several important differences do exist. It is essential to consider the differences that make pediatric cardiovascular management unique ( Table 51-1 ). Broadly, certain characteristics of the patient, congenital heart disease, and surgery account for these unique features and are reviewed here. These differences are attributable to normal organ system maturation in the neonate and young infant, differing pathophysiologic conditions in congenital heart disease, the diversity of surgical repairs, and the use of specialized CPB techniques such as deep hypothermia and total circulatory arrest.
Recognition of the changes associated with growth and development
is fundamental to understanding the pediatric patient. All the major organ systems
of the neonate and young infant undergo maturational changes that involve a dynamic
change in physiologic function. Only those
| Patient |
| Normal organ system development and maturational changes of infancy |
| Cardiovascular: blood flow patterns of circulation at birth, myocardial compliance, systemic and pulmonary vasculature, and β-adrenergic receptors |
| Pulmonary: respiratory quotient, closing capacity, chest compliance |
| Central nervous system: brain growth, cerebral blood flow, autonomic regulation |
| Renal: glomerular filtration rate, creatinine clearance |
| Hepatic: liver blood flow, microsomal enzyme activity |
| Disease-growth interrelationship |
| Effects of systemic disease alter somatic and organ growth |
| Compensatory ability of developing organs to recover from injury |
| Immunologic immaturity of the infant |
| Obligatory miniaturization (i.e., small patient size and body surface area) |
| Congenital heart disease |
| Diverse anatomic defects and physiologic changes |
| Altered ventricular remodeling owing to myocardial hypertrophy and ischemia |
| Chronic sequelae of congenital cardiac disease |
| Surgical procedures |
| Diversity of operations |
| Frequent intracardiac and right ventricular procedures |
| Use of deep hypothermia and circulatory arrest during repair |
| Trend toward repair in early infancy |
| Evolution of surgical techniques to avoid residua and sequelae |
| Trend toward wider application of certain operations |
The cardiovascular system changes markedly at birth because of a dramatic alteration in blood flow patterns ( Fig. 51-1 ).[2] During fetal life, blood flow returning to the right atrium bypasses the unventilated fluid-filled lungs. Blood is then preferentially shunted across the patent foramen ovale into the left atrium or passes from the right ventricle (RV) across the patent ductus arteriosus (PDA) to the systemic circulation. At birth, physiologic closure of the PDA and of the foramen ovale brings about the normal adult circulatory pattern. The presence of certain congenital heart defects or pulmonary disease can disrupt this normal adaptive process, creating a transitional circulation, in which right-to-left shunting persists across the foramen ovale or the PDA. Under such circumstances, the continued presence of a transitional circulation can lead to severe hypoxemia, acidosis, and hemodynamic instability, which are poorly tolerated in the neonate. In contrast, when initially treating some forms of congenital heart disease, the prolongation of this transitional circulation is actually beneficial, promoting systemic or pulmonary blood flow and postnatal viability. An example
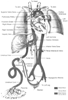 Figure 51-1
Course of the fetal circulation in late gestation. Note
the selective blood flow patterns across the foramen ovale and the ductus arteriosus.
Figure 51-1
Course of the fetal circulation in late gestation. Note
the selective blood flow patterns across the foramen ovale and the ductus arteriosus.
Another unique feature of the normal neonatal and infant cardiovascular system is the reduced myocardial reserve, compared with that in the healthy adult. The newborn left ventricular function is restricted by a reduced number of β-adrenergic receptors, high resting levels of circulating catecholamines, limited recruitable stroke work, an immature calcium transport system, and decreased ventricular compliance. [3] This limits contractile reserve and results in a left ventricle (LV) with a high level of resting tone.[4] Although the resting performance of the neonatal myocardium may be greater than in adults and older children, there is a greater sensitivity to β-blockade and only modest increases in cardiac performance following administration of the β-agonist drugs dobutamine and isoproterenol.[5]
On the ultrastructural level, a variety of cellular synthetic functions are occurring in immature myofibrils, which dominate the newborn heart. [4] Large nuclei, mitochondria, and surface membranes predominate within the myofibrils. In neonates, there is a 50% reduction in the number of myofibrils, and myofibrils are arranged in a nonlinear,
In addition to a reduced contractile mass, the calcium transport system in the neonatal myocardium is underdeveloped. The transverse tubular system is absent, and the sarcoplasmic reticulum, which has to store and release calcium, is small and inefficient. The neonatal heart is therefore more dependent on extracellular calcium levels than the adult myocardium.[9] [10] [11] [12] Because intracellular calcium concentrations play a central role in myocardial contractility, normal or even elevated plasma levels of ionized calcium may be necessary to augment or maintain an effective stroke volume.[13] This is in contrast to adult cardiac patients in whom calcium use during cardiac surgery has fallen into some disfavor, owing to direct concerns over myocardial ischemia and reperfusion injury.
Another unique feature relates to the pulmonary circulation. The pulmonary circulation undergoes significant changes during the first months of life. These changes are largely characterized by regression of the hypertrophied medial smooth muscular layer in the pulmonary arteries that exists in utero, resulting in a concomitant drop in pulmonary vascular resistance (PVR).[14] In the immediate newborn period, the large decrease in PVR is due to lung expansion and the vasodilatory effects of a higher PaO2 than existed in utero. Further decline in PVR throughout the next 2 months of life is attributable to regression of the smooth muscle layer in the pulmonary arterioles. A corresponding fall in pulmonary artery pressure occurs as PVR declines. Acute physiologic stress in the newborn period, such as hypoxemia or acidosis, can increase pulmonary artery pressure and thus PVR. If the resulting right ventricular hypertension causes reduced right ventricular compliance, right-to-left shunting can occur at the foramen ovale. Once PVR exceeds systemic vascular resistance (SVR), right-to-left shunting develops at the PDA. Either phenomenon will worsen the hypoxemia and eventually limit tissue oxygen delivery to the point of lactic acidosis. In contrast, left-to-right shunts, such as with a ventricular septal defect (VSD), produce intimal changes in the pulmonary vasculature and delay regression of medial muscle hypertrophy.[14] This results in persistent elevation of PVR.
Size differences between adult and pediatric cardiac patients require miniaturization. Anatomically, pediatric patients have small upper and lower airways, small veins and arteries, and a decreased body surface area, as compared with adult patients. There are several anesthetic implications related to patient size. Some centers believe that the placement of arterial catheters by cutdown in neonates and infants represents the most expedient approach, particularly when optimal sites are limited. Pulmonary artery catheters are used infrequently, both because of the technical difficulties in positioning the tip in the pulmonary artery and because of the fundamental fact that pulmonary flow bears no obligatory relationship to systemic output in children with either intra- or extracardiac communications. Transthoracic catheters for pressure monitoring and delivery of vasoactive substances are commonly placed from the surgical field. Adequacy of the repair and function can be assessed by transesophageal or epicardial echocardiography with Doppler color flow imaging.[15] [16] CPB serves as another example of the influence of patient size on management. The ratio of pump priming volume to patient blood volume is considerably higher in small children than in adults, resulting in a greater degree of hemodilution. Several studies have demonstrated a heightened inflammatory response to CPB in children, compared to adults.[17] [18] This effect is related to the disproportionate exposure to the nonendothelialized surfaces of the pump circuit per body surface area. Greater damage to the formed blood elements and plasma proteins is incurred, resulting in activation of mediators of inflammation.
Finally, in pediatric patients with congenital heart disease, the cardiovascular system often represents the sole cause of the medical problem. This is in contrast to the multiplicity of diagnoses and organ system involvement frequently found in adults with acquired cardiovascular disease. Moreover, there is a special disease-growth interrelationship unique to growing infants and children that does not exist in adults. This special interrelationship permits developing organs to compensate for and modify existing disease processes. Reparative and recuperative processes in children are greater as a result of this compensatory ability of developing organ systems. Adult cardiac patients unfortunately do not exhibit the same recuperative ability. Although children adapt well to cardiovascular pathology, there are some negative aspects to longstanding heart disease. Congenital heart disease has detrimental effects on somatic growth as well as on the growth and development of the brain, myocardium, and lung.
|
|
|
|
|
|
|
|
|
|
|
|
|