|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The marked array of anatomic and physiologic conditions seen with congenital heart disease distinguish these processes from acquired adult cardiac disease. The spectrum of intracardiac shunts, valve pathology (stenoses, regurgitation, or atresia), disrupted great artery connections, and the absence of one or more chambers of the heart preclude a uniform anesthetic approach to patients with congenital heart disease. Moreover, there are myocardial changes resulting from the hemodynamic impact and increased cardiac work incurred by these defects. Functionally, these myocardial changes place the ventricles at great risk for the development of intraoperative ischemia and failure. Therefore, an understanding of the isolated defect, associated myocardial changes, and hemodynamic consequences is fundamental to planning an appropriate anesthetic regimen.[6] Too often, because of the complexity and diversity of the defects, the anesthesiologist, cardiologist, and surgeon are focused on the specific anatomic considerations, and the physiologic implications are overlooked. Distilling congenital heart disease into a finite number of physiologic categories
Although the structural variations seen in congenital heart disease
constitute an encyclopedic list of malformations, anesthetic management is more logically
designed to achieve physiologic goals. A general physiologic classification is listed
in Table 51-2
. Fortunately,
although structurally complex, these defects can be understood in a more limited
physiologic spectrum. Identification and classification on the basis of physiology
provide an organized framework for the intraoperative anesthetic management and postoperative
care of children with complex congenital cardiac defects. In general, congenital
heart lesions fit into one of four categories: shunts, mixing lesions, flow obstruction,
and regurgitant valves (see Table 51-2
).
Each category imposes at least one of three pathophysiologic states: ventricular
volume overload, ventricular pressure overload, or hypoxemia. Ultimately, these
pathophysiologic conditions can result in myocardial failure or pulmonary vascular
disease. Medical and surgical perioperative management strategies should be focused
| Physiologic Classification | Pulmonary Blood Flow | Comments |
|---|---|---|
| Shunts |
|
|
| Left-to-right |
|
|
| VSD | ↑ | Volume-overloaded ventricle |
| ASD |
|
Develop CHF |
| PDA |
|
|
| AV canal |
|
|
| Right-to-left |
|
|
| Tetralogy of Fallot | ↓ | Pressure-overloaded ventricle |
| Pulmonary atresia/VSD |
|
Cyanotic |
| Eisenmenger complex |
|
Hypoxemia |
| Mixing lesions |
|
|
| Transposition/VSD | Generally | Variable pressure versus volume loaded |
| Tricuspid atresia | ↓ | Usually cyanotic |
| Anomalous venous return |
but variable |
|
| Univentricular heart |
|
|
| Obstructive lesions |
|
|
| Interrupted aortic arch |
|
Ventricular dysfunction |
| Critical aortic stenosis |
|
Pressure-overloaded ventricle |
| Critical pulmonic stenosis |
|
Ductal dependence |
| Hypoplastic left heart syndrome |
|
|
| Coarctation of the aorta |
|
|
| Mitral stenosis |
|
|
| Regurgitant lesions |
|
|
| Ebstein's anomaly |
|
Volume overloaded ventricle |
| Other secondary causes |
|
Develop CHF |
|
ASD, atrial septal defect; AV canal, atrioventricular canal;
CHF, congestive heart failure; PDA, patent ductus arteriosus; |
||
Shunts are intracardiac connections between chambers or extracardiac connections between a systemic and pulmonary artery; examples are an atrial septal defect (ASD), a VSD, and a PDA. The direction of blood flow through the shunt is dependent on the relative resistances on either side of the shunt and on the size of the shunt orifice.[19] With a nonrestrictive VSD or PDA that does not impede blood flowing freely in each direction, the main determinant of blood flow is the resistance of the pulmonary and systemic vascular beds. The direction and magnitude of shunt at the atrial level are governed by the relative differences in ventricular compliance and respective atrioventricular (AV) valve function. The effect that a shunt lesion has on the cardiovascular system depends on its size and direction, either right-to-left or left-to-right. Left-to-right shunts occur when the pulmonary vascular resistance is lower than the systemic vascular resistance and blood flow is preferentially directed toward the lungs, resulting in increased pulmonary blood flow.
In patients with large left-to-right shunts and low pulmonary vascular resistance, a substantial increase in pulmonary blood flow can occur. This results in three pathophysiologic problems: (1) congestion of the
Occasionally, the sudden increase in wall stress imposed on a dilated ventricle that must now pump solely against systemic vascular resistance can produce worsening ventricular failure during the early postoperative period after eliminating the low resistance "pop-off" into the pulmonary circulation. If the left-to-right shunt is not repaired, prolonged exposure to increased pulmonary blood flow results in progressive elevations in PVR. Fixed changes in pulmonary arterioles may occur, leading to pulmonary vascular obstructive disease. Table 51-2 lists common left-to-right shunt lesions.
Right-to-left shunts occur when pulmonary vascular or right ventricular outflow tract resistance exceeds systemic vascular resistance, thereby reducing pulmonary blood flow. The systemic circulation receives an admixture of deoxygenated blood via the shunt and manifests clinically as cyanosis and hypoxemia. Pure right-to-left shunting due to increased PVR is seen in the Eisenmenger complex and in persistent pulmonary hypertension of the newborn with atrial and ductal level shunting. More commonly, PVR is low, and a more complex lesion with obstruction of pulmonary outflow, proximal to the pulmonary vasculature, produces right-to-left shunting. Defects such as tetralogy of Fallot represent classic right-to-left shunts. The shunting occurs through the VSD because of pulmonary outflow obstruction. Systemic perfusion is generally normal with right-to-left shunting lesions unless hypoxemia becomes severe enough to impair oxygen delivery to tissue. Thus, there are two pathophysiologic problems: (1) reduced pulmonary blood flow resulting in systemic hypoxemia and cyanosis, and (2) an increased impedance to right ventricular ejection resulting in pressure overload, which may ultimately lead to ventricular dysfunction and failure of the RV. However, the physiologic mechanisms designed to compensate for pressure overload rarely create abnormalities in systolic or diastolic function early in the natural history of the disease process. In contradistinction to lesions that produce ventricular volume overload, ventricular dysfunction and failure typically take years to develop in the context of isolated pressure overload.
Mixing lesions constitute the largest group of cyanotic congenital
heart defects (see Table 51-2
).
In these defects, the mixing between the pulmonary and the systemic circulation
is so large that the systemic and pulmonary artery oxygen saturations approach each
other. The pulmonary-to-systemic flow ratio (![]() p/
p/![]() s) is independent of
shunt size and totally dependent on vascular resistance or outflow obstruction.
The pulmonary and systemic circulations tend to be in parallel with one another rather
than in series (see Table 51-2
).
In patients with no outflow obstruction, flow to the systemic or pulmonary circulation
is dependent on the relative vascular resistances of both circuits, such as with
univentricular hearts or double-outlet RV. If SVR exceeds PVR, as in the typical
circumstance, the tendency is toward excessive pulmonary blood flow, and the predominant
pathophysiologic process is left-to-right shunting. These patients have increased
pulmonary blood flow, ventricular volume overload, and a gradual elevation of PVR
over time. If PVR exceeds SVR, as may occur episodically in ductal-dependent lesions
such as hypoplastic left heart syndrome, systemic blood flow predominates and pulmonary
blood flow dramatically decreases, causing progressive hypoxemia ( Table
51-3
).
s) is independent of
shunt size and totally dependent on vascular resistance or outflow obstruction.
The pulmonary and systemic circulations tend to be in parallel with one another rather
than in series (see Table 51-2
).
In patients with no outflow obstruction, flow to the systemic or pulmonary circulation
is dependent on the relative vascular resistances of both circuits, such as with
univentricular hearts or double-outlet RV. If SVR exceeds PVR, as in the typical
circumstance, the tendency is toward excessive pulmonary blood flow, and the predominant
pathophysiologic process is left-to-right shunting. These patients have increased
pulmonary blood flow, ventricular volume overload, and a gradual elevation of PVR
over time. If PVR exceeds SVR, as may occur episodically in ductal-dependent lesions
such as hypoplastic left heart syndrome, systemic blood flow predominates and pulmonary
blood flow dramatically decreases, causing progressive hypoxemia ( Table
51-3
).
In patients with a mixing lesion and left ventricular outflow obstruction, pulmonary blood flow may be sufficiently excessive to impair systemic perfusion. In patients with mixing lesions and a right ventricular outflow obstruction such as a single ventricle with subpulmonic stenosis, systemic-to-pulmonary flow can vary from balanced flow to significantly decreased pulmonary blood flow in which the severity of hypoxemia depends on the degree of obstruction. Typical mixing lesions include truncus arteriosus, univentricular heart, total anomalous pulmonary venous return, pulmonary atresia with large VSD, and single atrium.
Obstructive lesions range from mild to severe. Severe lesions
present in the newborn period with a pressure-overloaded, diminutive, or profoundly
dysfunctional ventricle proximal to the obstruction. These lesions include critical
aortic stenosis, critical pulmonic stenosis, coarctation of the aorta, or interrupted
aortic arch. Although aortic and pulmonary atresia represent the most extreme variants
of outflow tract obstruction, they
| PDA Provides Systemic Flow | PDA Provides Pulmonary Flow |
|---|---|
| Coarctation of the aorta | Pulmonary atresia |
| Interrupted aortic arch | Critical pulmonary stenosis |
| Hypoplastic left heart syndrome | Severe subpulmonic stenosis with VSD |
| Critical aortic stenosis | Tricuspid atresia with pulmonic stenosis |
| PDA, patent ductus arteriosus; VSD, ventricular septal defect. | |
Pathophysiologic problems in critical neonatal left heart obstructive lesions include (1) profound left ventricular failure, (2) impaired coronary perfusion with an increased incidence of ventricular ectopy, (3) systemic hypotension, (4) PDA-dependent systemic circulation, and (5) systemic hypoxemia. The pathophysiologic problems of critical neonatal right heart obstructive lesions include (1) right ventricular dysfunction, (2) decreased pulmonary blood flow, (3) systemic hypoxemia, and (4) PDA-dependent pulmonary blood flow. Apart from the most extreme variants that become evident in the neonatal period, infants and children with outflow obstruction (e.g., mild to moderate aortic or pulmonary stenosis, coarctation of the aorta) manifest the effective compensatory mechanisms for pressure overload, often remaining clinically asymptomatic for many years.
Regurgitant valves are uncommon as primary congenital defects. Ebstein's malformation of the tricuspid valve is the only pure regurgitant defect presenting in the newborn period. However, regurgitant lesions are frequently associated with an abnormality of valve structure, such as incomplete or partial atrioventricular canal defect, truncus arteriosus, and tetralogy of Fallot with an absent pulmonary valve. The pathophysiology of regurgitant lesions includes (1) volume-overloaded circulation and therefore (2) progression toward ventricular dilation and failure.
When considering the incidence of all the congenital heart defects, three uncomplicated left-to-right shunts (VSD, ASD, PDA) and two obstructive lesions (pulmonic stenosis, coarctation) constitute 60% of all congenital cardiac defects. Mixing lesions, complicated obstructive defects, and right-to-left shunting lesions account for the vast majority of the remaining 40%. The latter group of defects, which are more difficult to manage, are more labor-intensive and have a significantly higher morbidity and mortality rate. This observation is directly attributed to the complexity of the cardiovascular abnormalities seen in this group, in which there is an absence of a chamber or a major ventricular-arterial connection.
The chronic effects of congenital heart disease are a consequence of the imposed hemodynamic stress of the defect or the residua and sequelae after cardiac surgery. These effects continue to alter normal growth and development of the cardiovascular system as well as other organ systems throughout life.[21] Complete surgical cures are rarely achieved, and some repairs are palliative rather than corrective; therefore, abnormalities before and after repair produce long-term effects in patients with congenital heart disease.[22] Although the overall outlook for these patients is good in most instances, every defect has associated myocardial changes, and every repair leaves certain obligatory abnormalities. Many of the abnormalities are trivial and have no major import. Others affect major organ system processes, such as ventricular function, central nervous system growth, the conduction system of the heart, or pulmonary blood flow. Under these circumstances, the long-term quality of life is affected. Whether anesthetizing these patients for their primary or subsequent cardiac repair or for noncardiac surgery, these chronic changes should be ascertained and reflected in the anesthetic plan.
The myocardium is continually remodeled by specific hemodynamic stresses in utero and throughout life. Right ventricular growth and development are influenced by the low-resistance afterload of the pulmonary circulation. The LV is coupled to the high-resistance systemic circulation, which accelerates its rate of growth and development. This situation gives rise to the adult condition in which left ventricular dominance of myocardial muscle mass occurs. This entire developmental process is referred to as dynamic ventricular modeling.[6] Abnormal hemodynamic loading conditions associated with congenital heart disease interrupt the normal ventricular modeling process ( Fig. 51-2 ).[23] Abnormal ventricular remodeling typically begins in utero and stimulates an increase in ventricular mass. Increased ventricular mass is due to both hyperplasia and hypertrophy of myocytes in response to altered wall stress on the developing ventricle. The resultant biomechanical deformation of the ventricle alters its geometry, affecting normal systolic and diastolic function.
Abnormalities of ventricular performance at rest and with exercise can be detected in patients with chronic hemodynamic overload and complex cyanotic lesions. These abnormalities in ventricular function are the consequences of chronic ventricular overload, repeated episodes of myocardial ischemia and residua or sequelae of surgical treatment (ventriculotomy, altered coronary artery supply, inadequate myocardial protection).[21] The physiologic adaptive responses to chronic hypoxemia and ventricular pressure or volume overload are the primary stimuli producing the long-term ventricular dysfunction. Although chronic volume overload of the LV as seen with left-to-right shunts or a chronic pressure-loaded LV due to left-sided obstructive lesions results in congestive heart failure, the compensatory mechanisms for pressure overload create less physiologic disturbance, particularly in diastolic function. Consequently, congestive heart failure occurs later in the natural history of
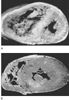 Figure 51-2
Comparison of ventricular hypertrophy patterns demonstrating
altered ventricular remodeling in two different congenital heart defects. A,
Note the right ventricular hypertrophy and the diminutive left ventricle (LV) in
tetralogy of Fallot. B, Note the severe left ventricular
hypertrophy and septal bulging into the right ventricle (RV) in aortic stenosis.
Figure 51-2
Comparison of ventricular hypertrophy patterns demonstrating
altered ventricular remodeling in two different congenital heart defects. A,
Note the right ventricular hypertrophy and the diminutive left ventricle (LV) in
tetralogy of Fallot. B, Note the severe left ventricular
hypertrophy and septal bulging into the right ventricle (RV) in aortic stenosis.
Initial manifestations of congestive heart failure reflect alterations in ventricular compliance that result from a variety of biophysical responses to abnormal loading conditions. The ventricular dilation and compensatory hypertrophy that accompany volume overload provide effective compensation to preserve normal systolic wall stress, but alterations in diastolic wall stress become evident ( Fig. 51-3 ).[20] Ultimately, chronic or severe pressure loads manifest similar changes as the resultant myocardial hypertrophy outgrows vascular supply and results in ischemia and fibroblast proliferation. Permanent changes in myocardial structure and function are the end result.
In patients with cyanotic conditions, the long-term compensation for chronic hypoxemia shows a major redistribution of organ perfusion with selected blood flow to the heart, brain, and kidney and decreased flow to the splanchnic circulation, skin, muscle, and bone. Chronic hypoxemia is associated with increased work of breathing in an attempt to increase oxygen uptake and delivery. The most dramatic complications are decreased rate of somatic growth, increased metabolic rate, and an increase in hemoglobin concentration seen in children with palliated or unrepaired defects.
|
|
|
|
|
|
|
|
|
|
|
|
|