Myocardial Ischemia
Not surprisingly, ECG changes are common after aortic cross-clamping
and anoxic cardiac arrest. After revascularization procedures, ECG changes have
been described as occurring in approximately 60% of patients.[284]
Although these studies were conducted in patients undergoing conventional revascularization
procedures, it is clear that ECG changes also develop when other techniques such
as OPCAB grafting and minimally invasive procedures are used. Moreover, ECG changes
also commonly occur in patients undergoing non-revascularization open heart procedures
such as valve replacement. The anesthesiologist should understand the possible causes,
especially those that might be remediable, assess the significance of the observed
ECG changes, and treat appropriately.
The possible causes of new-onset ECG changes are listed in Table
50-17
. A determination of the significance of new ECG changes after revascularization
is difficult but important. The most common ECG changes involve ST- and T-wave changes.
These changes often resolve over time and are not necessarily associated with an
increase in morbidity and mortality. However, some patients do progress to either
Q-wave changes or high levels of enzyme leak, both of which are known to be associated
with adverse outcomes.[284]
[285]
Both these changes take hours to develop, and the decision regarding the significance
of intraoperative ECG changes revolves around assessing the totality of the circumstances.
One must determine whether the patient is at high risk for ischemic changes to begin
with (e.g., severe distal coronary artery disease, thromboembolic risk, anastomotic
difficulties) and then determine whether the ECG changes are regional or generalized.
Regional ECG changes suggest a local problem such as a kinked graft, an anastomotic
issue, or an
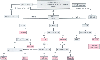 Figure 50-43
Suggested treatment algorithm for patients with excessive
post-cardiopulmonary bypass microvascular bleeding (MVB). Antifibrinolytic Rx, antifibrinolytic
therapy (e.g., epsilon-aminocaproic acid, tranexamic acid, aprotinin); CR, clot ratio
values (hemoSTATUS cartridge, Hepcon instrument); DDAVP, desmopressin acetate; D-dimers,
whole blood D-dimer assay (SimpleRED test); FFP, plasma therapy (2 U of fresh frozen
plasma); heparinase ACT, heparinase kaolin-activated clotting time test (ACT instrument);
heparinase aPTT, heparinase, activated partial thromboplastin time test (Coagucheck
Plus); MA, maximum amplitude (thromboelastograph); MA/A60
ratio, maximum
amplitude/amplitude at 60 minutes (thromboelastograph); [+] MVB, continued microvascular
bleeding; PF, platelet force measurements (Hemodyne Instrument); PLAT count, platelet
count (1000/µL); Platelets, platelet transfusion (6 U of random donor or apheresis
unit equivalent); PT:aPTT, prothrombin time and activated partial thromboplastin
time control values (values/mean values from a normal reference population); R2/R3,
R2 and R3 slope values (Sonoclot instrument); TT/HNTT, whole blood thrombin time/heparin-neutralized
thrombin time test (Hemochron instrument); WB FIB, whole blood fibrinogen test (Hemochron
instrument); WB HC, whole blood heparin concentration cartridge (Hepcon instrument).
(Redrawn from Despotis GJ, Joist JH, Goodnough LT: Monitoring hemostasis
with cardiac surgery: The impact of point-of-care testing on blood loss and transfusion
outcomes. Clin Chem 43:1684–1969, 1997.)
Figure 50-43
Suggested treatment algorithm for patients with excessive
post-cardiopulmonary bypass microvascular bleeding (MVB). Antifibrinolytic Rx, antifibrinolytic
therapy (e.g., epsilon-aminocaproic acid, tranexamic acid, aprotinin); CR, clot ratio
values (hemoSTATUS cartridge, Hepcon instrument); DDAVP, desmopressin acetate; D-dimers,
whole blood D-dimer assay (SimpleRED test); FFP, plasma therapy (2 U of fresh frozen
plasma); heparinase ACT, heparinase kaolin-activated clotting time test (ACT instrument);
heparinase aPTT, heparinase, activated partial thromboplastin time test (Coagucheck
Plus); MA, maximum amplitude (thromboelastograph); MA/A60
ratio, maximum
amplitude/amplitude at 60 minutes (thromboelastograph); [+] MVB, continued microvascular
bleeding; PF, platelet force measurements (Hemodyne Instrument); PLAT count, platelet
count (1000/µL); Platelets, platelet transfusion (6 U of random donor or apheresis
unit equivalent); PT:aPTT, prothrombin time and activated partial thromboplastin
time control values (values/mean values from a normal reference population); R2/R3,
R2 and R3 slope values (Sonoclot instrument); TT/HNTT, whole blood thrombin time/heparin-neutralized
thrombin time test (Hemochron instrument); WB FIB, whole blood fibrinogen test (Hemochron
instrument); WB HC, whole blood heparin concentration cartridge (Hepcon instrument).
(Redrawn from Despotis GJ, Joist JH, Goodnough LT: Monitoring hemostasis
with cardiac surgery: The impact of point-of-care testing on blood loss and transfusion
outcomes. Clin Chem 43:1684–1969, 1997.)
embolic event, whereas generalized changes might suggest difficulties with myocardial
protection. However, it is entirely possible that regional changes could also result
from inadequate myocardial protection in an area of myocardium distal to a critical
lesion, for example. Finally, one must determine whether the ECG changes are associated
with indices of deteriorating myocardial performance. Specifically, are the ECG
changes associated with dysrhythmias or with deteriorating function (as assessed
by TEE, filling pressures, and cardiac output)?
If it is determined that a specific cause is responsible for the
ECG changes, that cause is addressed directly, either surgically (e.g., redo an anastomosis)
or medically (e.g., treat presumed arterial conduit spasm with a calcium antagonist).
If a specific cause cannot be identified or if it is identified but cannot be addressed,
ischemia should be managed along conventional lines—that is, favorably manipulate
the determinants of myocardial oxygen supply and demand with pharmacologic adjuncts
and, if necessary, IABP counterpulsation.
 Figure 50-43
Suggested treatment algorithm for patients with excessive
post-cardiopulmonary bypass microvascular bleeding (MVB). Antifibrinolytic Rx, antifibrinolytic
therapy (e.g., epsilon-aminocaproic acid, tranexamic acid, aprotinin); CR, clot ratio
values (hemoSTATUS cartridge, Hepcon instrument); DDAVP, desmopressin acetate; D-dimers,
whole blood D-dimer assay (SimpleRED test); FFP, plasma therapy (2 U of fresh frozen
plasma); heparinase ACT, heparinase kaolin-activated clotting time test (ACT instrument);
heparinase aPTT, heparinase, activated partial thromboplastin time test (Coagucheck
Plus); MA, maximum amplitude (thromboelastograph); MA/A60
ratio, maximum
amplitude/amplitude at 60 minutes (thromboelastograph); [+] MVB, continued microvascular
bleeding; PF, platelet force measurements (Hemodyne Instrument); PLAT count, platelet
count (1000/µL); Platelets, platelet transfusion (6 U of random donor or apheresis
unit equivalent); PT:aPTT, prothrombin time and activated partial thromboplastin
time control values (values/mean values from a normal reference population); R2/R3,
R2 and R3 slope values (Sonoclot instrument); TT/HNTT, whole blood thrombin time/heparin-neutralized
thrombin time test (Hemochron instrument); WB FIB, whole blood fibrinogen test (Hemochron
instrument); WB HC, whole blood heparin concentration cartridge (Hepcon instrument).
(Redrawn from Despotis GJ, Joist JH, Goodnough LT: Monitoring hemostasis
with cardiac surgery: The impact of point-of-care testing on blood loss and transfusion
outcomes. Clin Chem 43:1684–1969, 1997.)
Figure 50-43
Suggested treatment algorithm for patients with excessive
post-cardiopulmonary bypass microvascular bleeding (MVB). Antifibrinolytic Rx, antifibrinolytic
therapy (e.g., epsilon-aminocaproic acid, tranexamic acid, aprotinin); CR, clot ratio
values (hemoSTATUS cartridge, Hepcon instrument); DDAVP, desmopressin acetate; D-dimers,
whole blood D-dimer assay (SimpleRED test); FFP, plasma therapy (2 U of fresh frozen
plasma); heparinase ACT, heparinase kaolin-activated clotting time test (ACT instrument);
heparinase aPTT, heparinase, activated partial thromboplastin time test (Coagucheck
Plus); MA, maximum amplitude (thromboelastograph); MA/A60
ratio, maximum
amplitude/amplitude at 60 minutes (thromboelastograph); [+] MVB, continued microvascular
bleeding; PF, platelet force measurements (Hemodyne Instrument); PLAT count, platelet
count (1000/µL); Platelets, platelet transfusion (6 U of random donor or apheresis
unit equivalent); PT:aPTT, prothrombin time and activated partial thromboplastin
time control values (values/mean values from a normal reference population); R2/R3,
R2 and R3 slope values (Sonoclot instrument); TT/HNTT, whole blood thrombin time/heparin-neutralized
thrombin time test (Hemochron instrument); WB FIB, whole blood fibrinogen test (Hemochron
instrument); WB HC, whole blood heparin concentration cartridge (Hepcon instrument).
(Redrawn from Despotis GJ, Joist JH, Goodnough LT: Monitoring hemostasis
with cardiac surgery: The impact of point-of-care testing on blood loss and transfusion
outcomes. Clin Chem 43:1684–1969, 1997.)