|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Massive hemoptysis[578] is uncommon and occurred in less than 0.5% of patients admitted to a large pulmonary medicine service. It has been arbitrarily defined, on the basis of the daily volume of blood expectorated, as 200 to 600 mL in 24 to 48 hours, or it is defined from the standpoint of causing acute airway obstruction or major hypotension.[578]
More than 90% of reported cases of massive hemoptysis have a chronic infectious cause[579] because chronic inflammation leads to profuse vascularization of the high-pressure bronchial artery system. Subsequently, any erosion or rupture of enlarged bronchial arteries will result in massive hemoptysis. Active tuberculosis is the most common and bronchiectasis the second most common infection causing massive hemoptysis.[580] The majority of the remaining cases of hemoptysis are due to bleeding neoplasms.
In a series of 55 pulmonary resections performed for massive hemoptysis (600 mL in 16 hours), a mortality rate of 18% was reported[581] ; this rate was markedly better than that with conservative treatment, which resulted in a mortality rate of 75% in patients who bled 600 mL or more in 16 hours and 54% in those who bled 600 mL or more in 48 hours.[582] [583] However, routine use of surgery has been debated inasmuch as other authors have found that somewhat lesser degrees of hemoptysis may be successfully managed conservatively regardless of the amount of bleeding in the first 24 hours.[578] Nevertheless, surgery (resection) is probably indicated in patients who require multiple transfusions, in those in whom bleeding results in progressive impairment of pulmonary function (aspiration should be evaluated by serial chest radiographs and arterial blood gas determinations), and in those in whom hemoptysis persists for several days despite optimum medical treatment. Contraindications to surgery include inoperable carcinoma of the lung, an inability to localize the bleeding site, and the presence of severe bilateral pulmonary disease and systemic disease (debilitation). These patients are candidates for bronchial artery embolization ( Fig. 49-40 ) (see later).
Bronchoscopy during active bleeding is the single most important technique for determining the cause and location of bleeding and should be performed in all patients; the procedure should be done in the operating room so that immediate resection can be performed (see Fig. 49-40 ).[583] Most surgeons use a rigid bronchoscope because of the much greater suctioning and ventilating capability. However, a flexible fiberoptic bronchoscope may be used if there is no active bleeding or the site of bleeding is thought to be in the upper lobes.
The surgeon may be able to control bleeding and the spread of bleeding during bronchoscopy (see Fig. 49-40 ). Topical iced saline and vasoconstrictors can be administered through the bronchoscope to control bleeding, provided that the bleeding is not so massive that it precludes visualization of its origin.[584] Control of the spread of blood from one lung to the other can be achieved with the use of a bronchial blocker (e.g., balloon-tipped Fogarty catheter) in the main bronchus of the bleeding side or the use of gauze packing of the bleeding segment or side. [578] The Nd:YAG laser has proved efficacious in the treatment of hemoptysis in patients with lung cancer.[585] [586] In a very exciting new development in pulmonary medicine, two reports describe complete cessation of bleeding without any adverse effect in almost all patients by selective intrabronchial spraying of fibrin precursors through a catheter in the suction port of a fiberoptic bronchoscope.[587] [588] During bronchoscopy, the surgeon should frequently restore adequate oxygenation and ventilation by intubating the uninvolved main stem bronchus. If the patient can withstand surgery and has an identified operable lesion, surgery should be performed. If the patient cannot withstand surgery or has an inoperable lesion, bronchial embolization should be attempted (see Fig. 49-40 ).
Anesthetic management of massive hemoptysis has several important preoperative priorities. For patients with
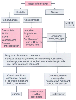 Figure 49-40
Treatment algorithm for massive hemoptysis. The most
important function of emergency bronchoscopy is to establish or diagnose the cause
and site of bleeding. In addition, the amount and spread of bleeding can be controlled
during emergency bronchoscopy (see items listed in the algorithm under "Diagnosis
of cause and treatment"). (Modified from Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-40
Treatment algorithm for massive hemoptysis. The most
important function of emergency bronchoscopy is to establish or diagnose the cause
and site of bleeding. In addition, the amount and spread of bleeding can be controlled
during emergency bronchoscopy (see items listed in the algorithm under "Diagnosis
of cause and treatment"). (Modified from Benumof JL: Anesthesia for Thoracic
Surgery. Philadelphia, WB Saunders, 1987.)
Coughing may increase bleeding. The advisability of using sedatives and cough suppressants is time dependent. In an unintubated patient, the ability to cough may be lifesaving, and suppressants should therefore be avoided. In an intubated patient, suctioning can replace the cough mechanism, and suppression of coughing may decrease bleeding. The patient's coagulation profile should be determined early; if any abnormalities are noted, they should be corrected. During and after bronchoscopy, the surgeon and pulmonologist can control bleeding by iced saline lavage, the use of topical vasoconstrictors, placement of a bronchial blocker or gauze packing, laser coagulation, fibrin seal, and the use of bronchial artery embolization (see earlier).
As soon as possible, several large-bore intravenous cannulas are inserted. The patient's blood is typed and cross-matched to ensure the availability of adequate amounts of blood products (whole blood, packed red blood cells, platelets, fresh frozen plasma). Transfusion should begin if appropriate. Antibiotics are administered preoperatively, and antituberculous drugs are started in patients with tuberculosis. Finally, the appropriate monitoring (e.g., arterial line, central venous line) is instituted. Many of these procedures should be undertaken simultaneously.
If a patient with massive hemoptysis does not have an indwelling endotracheal tube, preoxygenation should be instituted immediately. Adequate suctioning must be available. It may be necessary for the patient to be awake for intubation during massive, active, spontaneous bleeding to prevent the hazard of trying to visualize a blood-obscured airway in a paralyzed patient. Intubation performed in the semi-upright position may minimize the coughing that results in the presence of blood in the upper airway and may thereby provide a clearer field of vision.
If a patient without an indwelling endotracheal tube is to be anesthetized, aspiration precautions (e.g., cricoid pressure) should be used because many patients have swallowed expectorated blood. These patients are likely to be hypovolemic, so induction of anesthesia should be accomplished with a small dose of short-acting barbiturate or ketamine or with narcotics followed in rapid sequence by relaxation. If the larynx can be visualized, insertion of a DLT is preferable to insertion of a single-lumen tube. It should be remembered that if the patient is not actively bleeding but has a blood-filled cavity (i.e., a hemorrhagic lobe), this cavity will probably empty its contents into the dependent unsoiled lung when the patient is turned to the LDP; therefore, this situation is a strong indication for placement of a DLT. If a single-lumen
If a single-lumen tube is already in place, consideration should be given to conversion to a DLT, addition of a bronchial blocker, or achievement of an endobronchial position of the existing tube. Once the airway has been secured, the patient must be placed in the LDP with the bleeding lung in the nondependent position. Use of this position, of course, emphasizes the importance of separating the lungs. In all situations in which active tuberculosis is present or suspected, contamination precautions should be taken.
At the end of surgery, the endotracheal tube should be left in place and the patient ventilated mechanically. Most of these patients will have impaired gas exchange postoperatively as a result of preexisting lung disease, the likelihood that the nonbleeding lung has been soiled by the recent hemoptysis from the diseased lung, and the physiologic consequence of having just undergone a major anesthetic and surgical experience.
A bronchopleural fistula may be caused by rupture of a lung abscess, bronchus, bulla, cyst, or parenchymal tissue (as in the case of high levels of PEEP during mechanical ventilation) into the pleural space; by erosion of a bronchus by carcinoma or chronic inflammatory disease; and by breakdown of a bronchial suture line after pulmonary resection.
The diagnosis of bronchopleural fistula is usually made clinically. In early postpneumonectomy patients, the diagnosis is based on sudden dyspnea, subcutaneous emphysema, contralateral deviation of the trachea, and disappearance of the fluid level on radiographs of the chest. In patients after lobectomy, persistent air leak, purulent drainage, and expectoration of purulent material are usually diagnostic. When the fistula appears after removal of the chest tube, the diagnosis of a bronchopleural fistula is made on the basis of fever, purulent sputum, and a new air-fluid level in the pleural cavity on the chest radiograph. The diagnosis is confirmed by bronchoscopic examination in most, bronchography in a few, and sinograms (of the fistula) in occasional patients.[589] Other methods consist of injection of an indicator such as methylene blue into the pleural space and recovery of the indicator from sputum, accumulation of radionuclide in the pleural space after the inhalation of xenon, or a bronchogram showing spillage of contrast material into the vacant hemithorax.
In postpneumonectomy patients, if the disruption occurs early, it is possible to resuture the stump. Late postpneumonectomy bronchial disruption associated with empyema has been managed by conservative drainage, but definitive operative closure is now considered the treatment of choice.
In non-postpneumonectomy cases, if the lung expands to fill the thoracic cavity, the leak can usually be controlled with chest tube drainage alone. However, if the fistula is large and a significant leak through a large persistent pleural space occurs, it is unlikely that the fistula will close, and surgical resection is generally necessary.[589] An empyema complicating a bronchopleural fistula should, if possible, be drained before surgery (see the next section).
Finally, a spontaneous pneumothorax (no previous lung resection involved) is pathophysiologically similar to a bronchopleural fistula. Definitive surgical treatment for the management of spontaneous pneumothorax is indicated in three situations. First, surgery is required when conventional tube drainage and suction have been unsuccessful in clearing the pleural space and when, in effect, a bronchopleural fistula has formed. Second, surgical intervention is usually indicated when a second ipsilateral or first contralateral spontaneous pneumothorax occurs. Third, in view of the fact that spontaneous pneumothorax has a recurrence rate of 10% to 25%, if after the initial event the patient's lifestyle is such that a recurrence might be life threatening or highly inconvenient, definitive treatment is indicated. Surgical options are pleurectomy and chemical pleurodesis.[590]
Preoperatively, it is useful to estimate the loss of tidal volume through the bronchopleural fistula, which may be done in two ways. First, one should determine whether air bubbles intermittently or continuously through the chest tube. If air bubbles intermittently, the fistula is small. In contrast, when a patient has a large, low-resistance bronchopleural fistula or bronchial rupture, air may bubble continuously through the water seal chamber of the chest tube drainage system. Second, the size of the bronchopleural fistula may be quantitated by the difference between inhaled and exhaled tidal volumes. In a nonintubated patient, this may be determined with a tight-fitting mask and a fast-responding spirometer; in an intubated patient, it is determined by direct attachment of the spirometer to the endotracheal tube. The larger the leak, the greater the need to isolate the bronchopleural fistula (DLT, bronchial blocker).
Several nonsurgical approaches (use of various mechanical ventilation-chest tube drainage systems) have been used for the treatment of patients with bronchopleural fistula. These approaches consist of one-lung ventilation and differential lung ventilation, including HFV, PEEP to the pleural cavity equal to intrathoracic PEEP, and unidirectional chest tube valves.
For patients undergoing operative repair, the ability to deliver intraoperative positive-pressure ventilation adequately must be carefully considered preoperatively. If a fistula is obviously small, chronic, and uninfected, a standard endotracheal tube can probably be used safely. When in doubt, positive-pressure ventilation can be tested; if found inadequate, the standard endotracheal tube can be replaced with a DLT. During induction of anesthesia, suction on the chest tube should be discontinued to decrease the loss of tidal volume with the initiation of positive-pressure ventilation. If after the chest is opened, an excessive leak is encountered when using a standard endotracheal tube, ventilation can be improved by lung packing and manual control of the air leak.[591]
For large fistulas or fistulas of unknown size and for those in which an associated abscess or empyema is known or suspected to be present, intraoperative use of a
Pulmonary aspiration secondary to alcoholic stupor has classically been reported as the most common factor that precipitates lung abscess. Other historical factors that predispose patients to the formation of lung abscesses are abuse of other drugs, previous pneumonia, lung carcinoma, immunosuppression with steroid drugs, diabetes mellitus, the presence of a distant septic focus (hematogenous spread), and COPD.[596]
Empyema is the accumulation of pus in the pleural cavity. All the causes of a lung abscess described earlier may produce empyema. Empyema may also be caused by infection of residual clotted blood after a hemothorax (which was treated by chest tube placement) and after diagnostic thoracentesis, especially if a concurrent intra-abdominal injury or infection is present.[597] Both lung abscess and empyema may erode a bronchus and cause a bronchopleural fistula (see earlier).
Simple empyemas (without abscess) may be treated by repeat thoracentesis, tube thoracostomy, thoracoscopy, or open drainage with rib resection.[598] In any patient who does not improve with any of these therapies, an open drainage procedure followed by either lobectomy or segmentectomy is required.[599] [600]
If a surgical procedure is to be performed under general anesthesia in a patient with either a lung abscess or empyema, DLT intubation is absolutely indicated to prevent contamination of the uninfected lung by the infected lung. In addition, if empyema is to be treated by thoracoscopy, collapse of the diseased lung greatly aids access to the empyema.[601] [602] The seal of the endobronchial cuff should be tight and can be quantitated by a bubble-under-water technique. The position of the tube should be determined by fiberoptic bronchoscopy. Both these maneuvers should be completed before the patient is turned to the LDP.
During the surgical procedure, the diseased side should be suctioned frequently (fiberoptically if necessary). Whenever possible but particularly at the end of the procedure, the diseased lung should be fully expanded manually. When the lung has been fully expanded, the surgeon should check carefully for the presence of a bronchopleural fistula. The pleural cavity may be irrigated with antibiotics at the end of the procedure, and one must be mindful of the neuromuscular blockade effects of antibiotics.
|
|
|
|
|
|
|
|
|
|
|
|
|