|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tracheal resection is indicated, if technically feasible, in patients who have tracheal obstruction as a result of a primary tracheal tumor (most are carcinomas), previous tracheal trauma (e.g., stenosis from prolonged intubation), congenital anomalies, and vascular lesions. For patients who have operable tumors, approximately 80% undergo segmental resection (may include the carina or larynx) with primary anastomosis, 10% undergo segmental resection with prosthetic reconstruction, and in 10% a T-tube stent is inserted. Adjuncts to surgical extirpation include preoperative and postoperative external irradiation, internal radioactive seed irradiation (transferred by an endobronchial catheter or directly placed by thoracotomy), and preoperative laser debulking therapy.
Many previous technical limitations to the performance of tracheal surgery can now be overcome by careful preoperative delineation of the site and degree of obstruction, close intraoperative communication between the surgeon and anesthesiologist, improved anesthetic management techniques, and meticulous postoperative care. All these components contribute to the ability to provide adequate ventilation throughout the perioperative period. Although the results of this very complicated surgery on primary tracheal tumors depend on the tumor cell type, location, and method of resection, it is generally accepted that in the few institutions that have a reasonable degree of surgical experience, a worthwhile survival rate can be achieved in most patients. This section discusses the care of patients undergoing tracheal resection and describes several different methods of airway management (see Chapter 42 and Chapter 65 ). [512] [513] [514] [515] [516] [517] [518] [519] [520] [521] [522] [523] [524] [525] [526] [527] [528] [529] [530] [531] [532] [533] [534] [535] [536]
Unless airway obstruction is imminent, pulmonary function should be routinely studied preoperatively.
During surgery, all patients should have an arterial catheter placed to facilitate analysis of arterial blood gases. It should be placed in the left radial artery because the innominate artery (which supplies the right radial artery) crosses the trachea and may be compressed during surgery. A variety of methods for providing adequate oxygenation and elimination of carbon dioxide have been used during tracheal resection. These methods can be divided into five approaches: (1) standard orotracheal intubation, (2) insertion of a tube into the opened trachea distal to the area of resection, (3) HFJV through the stenotic area, (4) HFPPV, and (5) cardiopulmonary bypass.
The first technique uses a standard, but uncut long orotracheal tube that is placed above the tracheal lesion after induction of general anesthesia and is merely
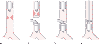 Figure 49-35
Airway management and surgical procedure for resection
of a high tracheal lesion. A, Initial intubation
above the lesion. B, Second endotracheal intubation
distal to the lesion after the trachea has been opened. C,
Placement of sutures for the posterior anastomosis. D,
The second endotracheal tube has been removed, and the original endotracheal tube
has been advanced distal to the anterior anastomosis. (Modified from Geffin
B, Bland J, Grillo HC: Anesthetic management of tracheal resection and reconstruction.
Anesth Analg 48:884, 1969.)
Figure 49-35
Airway management and surgical procedure for resection
of a high tracheal lesion. A, Initial intubation
above the lesion. B, Second endotracheal intubation
distal to the lesion after the trachea has been opened. C,
Placement of sutures for the posterior anastomosis. D,
The second endotracheal tube has been removed, and the original endotracheal tube
has been advanced distal to the anterior anastomosis. (Modified from Geffin
B, Bland J, Grillo HC: Anesthetic management of tracheal resection and reconstruction.
Anesth Analg 48:884, 1969.)
To overcome these problems, endotracheal or endobronchial tubes have been inserted into the opened trachea distal to the site of resection (second approach).[515] [519] [520] [521] [522] [523] [524] [525] With this second approach, initially either a small endotracheal tube is passed distal to the obstruction or a standard endotracheal tube is placed proximal to it (see Fig. 49-35A , Fig. 49-36A , and Fig. 49-37A ). All further tracheal and endobronchial intubations are performed with armored tubes passed into the airway, which is surgically opened distal to the lesion. The surgeon must have a complete set of sizes of endotracheal tubes to choose from because either a main stem bronchus or any lobar bronchus may need to be intubated.
With a high tracheal lesion, a cervical incision, possibly combined with a median sternotomy, provides adequate surgical exposure. An opening is made in the trachea distal to the area to be resected, and a sterile endotracheal tube is inserted by the surgeon into the distal part of the trachea (see Fig. 49-35B ).[515] [519] [520] This second endotracheal tube is connected to a Y-piece and a second set of anesthetic hoses and handed off to the anesthesiologist to continue ventilation. After excision of the tracheal lesion and placement of the posterior tracheal sutures, the second (distal) endotracheal tube is removed from the trachea, the original (first) endotracheal tube is advanced
With a low tracheal lesion, a right thoracotomy provides the necessary surgical exposure. If there is sufficient trachea distal to the area of resection, a Foley catheter with the tip cut off just distal to the balloon may be used as a single-lumen endotracheal tube. It is inserted by the surgeon and secured just above the carina, thereby avoiding endobronchial intubation and the need for one-lung anesthesia. [521] Otherwise, if not enough distance remains between the tracheal lesion and carina to provide placement of even this homemade endotracheal tube, endobronchial intubation and one-lung ventilation are necessary (see Fig. 49-36B ).[522] [523] [524] If oxygenation or ventilation is inadequate, it may be possible to decrease blood flow to the atelectatic lung by tightening reversible snares around the pulmonary artery of the nonventilated lung[515] [516] ; however, this maneuver may be technically difficult. An alternative technique is to pass a second endobronchial tube into the other bronchus to provide ventilation to both lungs (see later).[524] As with a high tracheal lesion, after the posterior anastomosis is completed, the endobronchial tube or tubes are removed and the original endotracheal tube pushed past the site of resection; in this situation, however, it is likely that endobronchial intubation may again be required to complete the anastomosis (see Fig. 49-36C and D ).
Several methods have been described for managing the airway during carinal resection.[515] [516] [534] While the affected segment is being resected, left lung ventilation may be carried out by endobronchial intubation of the left main stem bronchus below this lesion (see Fig. 49-37B ).[515] After the right main stem bronchus and
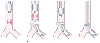 Figure 49-36
Airway management and surgical procedure for resection
of a low tracheal lesion. A, Initial intubation above
the lesion. B, Left endobronchial intubation distal
to the lesion after the trachea has been opened. C,
Placement of sutures for the posterior anastomosis. D,
The endobronchial tube has been removed, and the original endotracheal tube has been
advanced distal to the anterior anastomosis into an endobronchial position. (Modified
from Geffin B, Bland J, Grillo HC: Anesthetic management of tracheal resection and
reconstruction. Anesth Analg 48:884, 1969.)
Figure 49-36
Airway management and surgical procedure for resection
of a low tracheal lesion. A, Initial intubation above
the lesion. B, Left endobronchial intubation distal
to the lesion after the trachea has been opened. C,
Placement of sutures for the posterior anastomosis. D,
The endobronchial tube has been removed, and the original endotracheal tube has been
advanced distal to the anterior anastomosis into an endobronchial position. (Modified
from Geffin B, Bland J, Grillo HC: Anesthetic management of tracheal resection and
reconstruction. Anesth Analg 48:884, 1969.)
It is apparent that the conventional techniques of airway management for tracheal resection are fraught with hazard. During surgery, a slight head-down tilt helps minimize aspiration of blood and secretions. Intermittent sighs help prevent bronchiolar obstruction and atelectasis. High FIO2 is used because an oxygen-filled FRC permits a few extra minutes to correct relatively common episodes of airway obstruction or tube displacement (or both). Ventilation is continuously monitored by pulse oximetry, capnography, auscultation and observation of the chest, measurement of compliance (peak
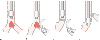 Figure 49-37
Airway management and surgical procedure for resection
of a carinal lesion. A, Initial intubation above
the lesion. B, Left endobronchial intubation distal
to the lesion after the left main stem bronchus has been severed. C,
The trachea is anastomosed to the right main stem bronchus. D,
The left endobronchial tube has been removed to allow for anastomosis between the
trachea and left main stem bronchus. Ventilation is accomplished through the original
endotracheal tube. (Modified from Geffin B, Bland J, Grillo HC: Anesthetic
management of tracheal resection and reconstruction. Anesth Analg 48:884, 1969.)
Figure 49-37
Airway management and surgical procedure for resection
of a carinal lesion. A, Initial intubation above
the lesion. B, Left endobronchial intubation distal
to the lesion after the left main stem bronchus has been severed. C,
The trachea is anastomosed to the right main stem bronchus. D,
The left endobronchial tube has been removed to allow for anastomosis between the
trachea and left main stem bronchus. Ventilation is accomplished through the original
endotracheal tube. (Modified from Geffin B, Bland J, Grillo HC: Anesthetic
management of tracheal resection and reconstruction. Anesth Analg 48:884, 1969.)
In an effort to overcome these problems, a third approach to airway management during tracheal resection was developed that consists of HFJV through small-bore endotracheal tubes or catheters.[525] [526] [527] [528] [529] [533] With this technique a small-bore uncuffed catheter is placed through the stenotic area, and ventilation is accomplished by exposing the lung to rapid intermittent, high-flow, fresh gas through the catheter. Oxygen jets entrain ambient air, which in turn provides the volume necessary for adequate ventilation. With this technique, acceptable blood gas values have been maintained, and there have been no deleterious effects on the circulation. With only a small catheter in the field, the surgeon can more easily perform the tracheal resection and anastomosis. Disadvantages of this technique, however, include possible inadequate escape of air around the jet catheter during exhalation when the catheter is passed through a tight stenotic area, plugging of the catheter with blood, displacement of the catheter, aspiration of blood, and technical difficulties with high-pressure injectors.
The fourth approach to airway management during tracheal resection involves the use of HFPPV.[514] This technique uses small tidal volumes (50 to 250 mL) delivered through a small catheter at relatively rapid rates (50 to 150/min). Advantages of using HFPPV for tracheal resection include a relatively unobstructed surgical field, no interruption of ventilation during surgery, minimized contamination of the lungs from blood and debris by a continuous outflow of gas, and minimized lung and mediastinal movement. In addition, production of CPAP lessens the risk of alveolar collapse. This technique has been used successfully by others for both tracheal[512] [513] and carinal resection.[512] During carinal operations, HFPPV to the left lung alone generally provides adequate oxygenation and ventilation, although a system of two catheters and bilateral HFPPV could be used if necessary. [514]
The fifth approach to airway management during tracheal resection, especially in cases involving carinal resection, has been the institution of cardiopulmonary bypass either at the time of resection[530] or before the start of surgery.[531] After resection, anesthesia can be continued by conventional techniques through a standard endotracheal tube. Although many surgical teams perform difficult tracheal resections with the cardiopulmonary bypass team standing by, the risk of intrapulmonary hemorrhage as a result of heparinization precludes its use in most cases.[515]
Helium-oxygen breathing mixtures can significantly decrease the resistance to gas flow through stenotic areas and may be considered preoperatively for some of these patients.[531] However, the use of helium-oxygen mixtures prevents the use of high FIO2 during anesthesia, and it is therefore not often recommended.[515]
Postoperatively, most patients are kept in a position of head flexion to reduce tension on the suture line. If ventilatory support is necessary postoperatively, the endotracheal tube must be positioned so that the cuff does not rest on any suture line. Early extubation is highly desirable to minimize the compromise in blood flow to the trachea that might be caused by an inflated tracheal cuff. Chest physiotherapy to remove secretions should not be
A bulla is defined as an air-filled, thin-walled space within the lung that results from the destruction of alveolar tissue. The walls of bullae are formed by connective tissue septa, compressed lung parenchyma, or pleura. A bulla usually represents a local end-stage area of emphysematous destruction. As separate but related entities, air cysts in the lung have their own epithelial margins, and they may also be associated with COPD[537] or be found in the absence of other pulmonary pathology.[538]
Bullectomy is the surgical resection of one or more bullae and is performed only in selected patients. The most important physiologic effect of bullae is compression of surrounding lung, and release of lung compression after bullectomy is considered to be the most important factor in relief of symptoms (rather than eliminating dead-space ventilation). Indications for bullectomy in COPD patients include intolerable breathlessness even after full medical therapy, rapidly enlarging bullae, and repeated occurrence of pneumothorax.[539] Patients with otherwise healthy lungs may undergo removal of a giant air cyst or bulla if it compresses a large area of normal lung and is causing functional impairment. Compression of a large area of lung can be visualized on the chest roentgenogram (as well as on an angiogram) as crowding together of pulmonary vessels. A strong case for functional impairment can be made if radioisotope studies show that the compressed area has good perfusion and some, but reduced ventilation.[540] [541]
The goal of surgery is to remove the bullae while preserving as much functioning lung as possible. Therefore, lobectomy is not only undesirable but often unnecessary. Patients considered too compromised to tolerate an open approach may tolerate a closed thoracoscopic approach; the reason that thoracoscopic carbon dioxide laser ablation of bullae has been so successful is that it maximizes preservation of normal lung better than any open thoracotomy approach does. It is likely that this approach to bullectomy will be used with increasing frequency in the next few years.[542]
Anesthesia for the removal of bullae involves several specific ventilation hazards. First, most of these patients have severe generalized chronic lung disease with little or no ventilatory reserve. Thus, ventilation (which must be controlled once the chest is opened) of one severely diseased lung (the one without the giant bullae) may be hazardous and entails the risks of hypoxemia, hypercapnia, and pneumothorax on the ventilated side. If the ventilated lung also contains bullae, the risks are obviously even greater. In addition, because general anesthesia is necessary for the procedure, most patients with severe lung disease will be committed to at least a short period of postoperative mechanical ventilatory support. Second, when a bulla or air cyst is in communication with a bronchus, positive-pressure ventilation may cause it to increase in size.[543] If a significant portion of the tidal volume enters the bullous cavity, alveolar dead-space ventilation will be greatly increased, and unless there is an equivalent increase in minute ventilation, the rest of the lung may be inadequately ventilated. This complication is most likely to occur when the chest is opened because the chest wall no longer limits expansion of the bulla. Third, because of the rapidity with which closed air spaces take up nitrous oxide and expand in size,[544] this anesthetic is best avoided (especially in patients whose bullae are thought to have poor communication with the bronchial system).[545] Fourth, if a check valve is present in the airway that communicates with the cavity, overinflation and air trapping may occur within the cavity. Fifth, positive pressure within a bulla might cause it to rupture and create a pneumothorax, which would probably be under tension if the chest were closed (especially in patients whose bullae are thought to have good communication with the bronchial system).[543] Tension pneumothorax in these patients is usually a catastrophic event because of impairment of venous return and cardiac output, as well as further compromise of ventilation. Insertion of a chest tube at this point would create, in effect, a large bronchopleural cutaneous fistula that could divert much of the ventilation out through the chest tube. HFV with low tidal volume and airway pressure has been used successfully to prevent positive-pressure rupture of a bulla according to a 1985 report.[546] Sixth, after bullectomy, the remaining lung may be so overexpanded (as a result of residual bullae or bronchospasm, or both) that considerable force is required to "stuff" the lung back into the hemithorax. The resultant obstruction of venous return and physical encroachment on the heart may require the use of inotropes to maintain adequate hemodynamics. Seventh, it is common for multiple small air leaks to be present after bullectomy. Collectively, these leaks cause considerable loss of delivered tidal ventilation through a chest tube. Effective ventilation and oxygenation are accomplished by trial and error using a variety of tidal volumes, airway pressures, inspiratory-expiratory ratios, flow rates, and ventilation rates or, alternatively, by HFV.
The cornerstone of anesthetic management of patients with giant bullae or cysts is insertion of a DLT to allow differential treatment of the two lungs. Thus, in patients with unilateral disease, a DLT can permit adequate ventilation of the nondiseased side while preventing rupture of the diseased side. In patients with bilateral disease, a DLT still allows differential lung treatment to maximize gas exchange, as well as providing an increased capability to deal with the complications of a ruptured bulla. For example, a DLT allows delivery of all possible permutations of HFV, CPAP, PEEP, and ZEEP to the two lungs, depending on the pathology in each lung. In addition, as each bulla is resected, a DLT permits ventilation to the operated lung to be reestablished for short periods,
A DLT can be inserted either with the patient awake and the airway topically anesthetized (for those with histories of repeated pneumothorax or severe bilateral bullae, or both) or with the patient under general anesthesia (for most patients) to isolate the affected lung and provide positive-pressure ventilation to the contralateral lung.[547] [548] While the depth of general anesthesia is being increased, spontaneous ventilation may be maintained (primarily indicated in patients with a history of repeated pneumothorax or bilateral bullae), but it should be realized that spontaneously breathing patients with significant pulmonary disease who are under general anesthesia may not be able to ventilate themselves adequately. Alternatively and preferably in most patients, the patient can be anesthetized and both lungs ventilated by using a limited amount of positive-airway pressure; gentle ventilation by hand is the best way to ensure low airway pressure. If a major air leak develops intraoperatively while positive-pressure ventilation is being delivered with a volume-cycled ventilator, inadequate ventilation may occur (the machine delivers the preset volume but it does not go into the lungs). Under these circumstances, a pressure-cycled high-inspiratory flow ventilator must be used (e.g., Siemens Servo 900C, Solna, Sweden).[542]
If limited positive-pressure ventilation is chosen, it is important that the anesthesiologist be able to diagnose and treat a pneumothorax rapidly. External stethoscopes should be attached over each hemithorax at the points where breath sounds are maximal to monitor for pneumothorax on each side.[549] However, advanced bullous disease may completely prevent breath sounds from being heard externally at all. In addition to a decrease in breath sounds, a pneumothorax or check valve mechanism in a cyst may be signaled by an increase in airway pressure, tracheal shift to the opposite side, or hypotension disproportionate to the depth of anesthesia. Thus, equipment for chest tube placement must be immediately available for these patients.
Theoretically, after bullectomy the patient's pulmonary status should be improved because the "healthy" lung tissue that was previously compressed by the bullae should now be able to expand. However, in our experience and that of others,[542] the weaning and extubation process often takes up to several days in patients with advanced disease. When mechanical ventilation is required postoperatively, positive airway pressure should again be minimized to decrease the possibility of producing a pneumothorax from rupture of suture lines or residual bullae[550] (or both) and to minimize air leaks from the remaining lung tissue.
When bilateral bullectomy is performed because of extensive disease in both lungs, a sternal-splitting incision with the patient supine is usually made. [551] However, sequential posterolateral thoracotomies may be planned if it is desired to see how the patient responds to the first bullectomy. With bilateral bullectomy, the same anesthetic principles apply as with unilateral bullectomy.
Advanced emphysema is a debilitating lung disease that afflicts 2 million people in the United States.[552] In emphysema, the lungs are hyperinflated with poor elastic recoil, the airways are dynamically compressed, the flattened diaphragms contract poorly, and cardiac filling may be impaired. These pathophysiologic changes result in hypoxemia, hypercapnia, increased work of breathing, and a debilitating shortness of breath. The goal of lung volume reduction surgery is to partially reverse these pathophysiologic mechanisms. [553]
Current approaches to bilateral lung volume reduction surgery include median sternotomy and thoracoscopy (with the use of either a linear cutting stapler or laser ablation) and reinforcement of incised lung parenchyma with exogenous buttressing materials (such as bovine pericardial strips).[553] The lung tissue to be resected is determined by visual assessment of the most diseased lung (which remains inflated during contralateral one-lung ventilation); it most often consists of multiple small regions, is nonanatomic, and usually involves 20% to 30% of that lung.[553] In some patients, however, only a few large blebs or bullae are resected. In either case, the most abnormal-appearing tissue is resected to allow expansion of the more normal remaining lung tissue. Most patients who have undergone this operation have experienced improvement in lung function and quality of life. The two principal causes of morbidity and mortality in patients undergoing this procedure are (1) respiratory impairment as a result of induction of general anesthesia and the trauma of the operative incision in these already critically ill patients and (2) complications arising from resection of emphysematous lung tissue, most notably persistent air leaks.
The overriding goal of anesthetic management is to have the patient adequately and quietly breathing spontaneously as soon as possible at the end of surgery to minimize positive pressure-induced lung air leaks. The main components of anesthetic management that allow achievement of this goal are the use of general anesthesia supplemented with thoracic epidural anesthesia intraoperatively and continued after surgery to provide postoperative analgesia, minimal to no intravenous narcotics, and fluid restriction; frequently, emergence and recovery are prolonged (can be a few hours), and mask ventilation assistance is required in the operating room.
Essential invasive monitoring consists of arterial and central venous catheters. The usual indications for a pulmonary artery catheter remain. A thoracic epidural catheter is placed before induction of general anesthesia and the position of the catheter is determined pharmacologically or fluoroscopically (or by both means). Optimally, the catheter tip is at an upper thoracic (T3-5) level, located in the mid-dermatome area of the incision. These very respiratory debilitated patients are either unpremedicated or receive only small doses of midazolam if they are anxious. Anesthesia is induced intravenously (without narcotics), the patient is paralyzed,
Unilateral bronchopulmonary lavage, or massive irrigation of the tracheobronchial tree of one lung, has been used with good success in patients with pulmonary alveolar proteinosis as a means of removing the enormous accumulations of alveolar lipoproteinaceous material characteristically present in these patients. [555] [556] [557] [558] [559] The lipoproteinaceous material is thought to be surfactant,[560] and the abnormal accumulation is due to failure of clearance mechanisms rather than enhanced formation.[561] The abnormal accumulation of alveolar lipoproteinaceous material is bilateral and symmetric and causes the classic chest radiographic picture of air space consolidation with patchy, poorly defined shadows throughout the lung.[562] The radiographic picture reflects the course of the disease. The air space consolidation causes progressive hypoxemia and shortness of breath (first on exertion and then at rest),[562] [563] and the lungs have low compliance. The diagnosis of alveolar proteinosis is made by correlating the clinical, radiologic, and laboratory data with the results of a lung biopsy. The indication for lung lavage is a PaO2 less than 60 mm Hg at rest or hypoxemic limitation of normal activity.[564] [565] Infrequently, lung lavage may be performed in patients with asthma, cystic fibrosis, and inhalation of radioactive dust.[552] [557] [565] [566] [567]
Unilateral lung lavage is performed under general anesthesia with a DLT to allow lavage of one lung while the other is ventilated ( Fig. 49-38 ). In patients with alveolar proteinosis, lavage is performed on one lung and then, after a few days' rest, on the other lung. After lung lavage these patients usually have marked subjective improvement that correlates with increases in PaO2 during rest and exercise, increased vital capacity and diffusion capacity, and clearing of the chest radiograph.[568] [569] Some
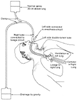 Figure 49-38
Technique for providing unilateral bronchopulmonary lavage.
A left clear plastic double-lumen endotracheal tube allows ventilation to the left
lung during lavage of the right lung (and vice versa). Normal saline is infused
and drained by gravity; clamps on the connection tubes determine the direction of
fluid flow. (See the text for details.) (From Benumof JL: Anesthesia for
Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-38
Technique for providing unilateral bronchopulmonary lavage.
A left clear plastic double-lumen endotracheal tube allows ventilation to the left
lung during lavage of the right lung (and vice versa). Normal saline is infused
and drained by gravity; clamps on the connection tubes determine the direction of
fluid flow. (See the text for details.) (From Benumof JL: Anesthesia for
Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
This section discusses the technique for anesthetic management
of bronchopulmonary lavage in patients with pulmonary alveolar proteinosis.[260]
When the patient is admitted to the hospital, V̇/![]() scans of the lung are
obtained. Ventilation can be maximized during lung lavage by performing the first
lavage on the most severely affected lung and allowing the "better" lung to provide
gas exchange. If the scan indicates relatively equal involvement (as is usually
the case), the left lung is lavaged first, with the larger right lung left to support
gas exchange.
scans of the lung are
obtained. Ventilation can be maximized during lung lavage by performing the first
lavage on the most severely affected lung and allowing the "better" lung to provide
gas exchange. If the scan indicates relatively equal involvement (as is usually
the case), the left lung is lavaged first, with the larger right lung left to support
gas exchange.
After several minutes of preoxygenation (see later), general anesthesia is induced with thiopental in divided doses and inhalation of either isoflurane or halothane in 100% oxygen. Isoflurane is relatively indicated in patients in whom therapeutic levels of theophylline (and the risk of arrhythmias) are present. Neuromuscular blockade is induced with a nondepolarizing muscle relaxant. When a suitable level of anesthesia has been
The question of patient position during unilateral lung lavage is important, for each position has major advantages and disadvantages ( Table 49-21 ). The LDP with the lavaged lung dependent minimizes the possibility of accidental spillage of lavage fluid from the dependent lavaged lung to the nondependent ventilated lung. However, during periods of lavage fluid drainage, pulmonary blood flow, which is gravity dependent, would preferentially perfuse the nonventilated dependent lung, and the right-to-left transpulmonary shunt would be maximal. An LDP with the lavaged lung nondependent minimizes blood flow to the nonventilated lung but, on the other hand, increases the possibility of accidental spillage of lavage fluid from the lavaged lung to the dependent ventilated lung. As a compromise, the supine position is used to balance the risk of aspiration with the risk of hypoxemia.
After insertion and checking of the DLT and positioning of the
patient, baseline total and individual lung compliance is measured. Airway pressure
can be electronically transduced and continuously recorded on a paper printout, and
a Wright spirometer should be placed in the expiratory limb of the anesthesia circle
system to measure tidal volume accurately. A volume ventilator
| Lateral decubitus with the lavaged lung nondependent |
| Advantage: Minimizes blood flow to the nonventilated lung |
| Disadvantage: Maximizes the possibility of spillage |
| Lateral decubitus with the lavaged lung dependent |
| Advantage: Minimizes the possibility of spillage |
| Disadvantage: Maximizes blood flow to the nonventilated lung |
| Supine position |
| Balances spillage and blood flow distribution problems |
Patients are completely preoxygenated before induction of anesthesia and lavage for two reasons. First, as with induction of general anesthesia in any patient, an oxygen-filled FRC greatly minimizes the risk of hypoxemia during the apneic period required for laryngoscopy and endotracheal intubation. This consideration has added importance for patients with alveolar proteinosis because they are already severely hypoxemic. Second, preoxygenation eliminates nitrogen from the lung that is to be lavaged. The alveolar gas is then composed of only oxygen and carbon dioxide. During fluid filling, these gases will be absorbed, which allows the lavage fluid maximal access to the alveolar space. Failure to remove nitrogen from the lung before filling the lavage fluid may leave peripheral nitrogen bubbles in the alveoli and thus limit the effectiveness of the lavage.
Warmed isotonic saline is used as the lavage fluid and is infused by gravity from a height of 30 cm above the midaxillary line. After the lavage fluid ceases to flow (i.e., lung filling is complete), drainage is accomplished by clamping the inflow line and unclamping the drainage line, which runs to a collection bottle placed 20 cm below the midaxillary line (see Fig. 49-38 ). The fluid inflow and outflow lines are connected to the appropriate endotracheal tube lumen by a Y-adapter. Each tidal lavage filling is accompanied by mechanical chest percussion and vibration of the lavaged hemithorax before drainage. The lavage fluid that is drained is typically light brown, and the sediment layers out at the bottom of the collection bottle after a short time. The process of filling and drainage of approximately 500- to 1000-mL aliquots is repeated until the lavage effluent clears (see Fig. 49-38 ). Volumes delivered to and recovered from each tidal lavage are recorded. Total lavage fluid volumes of 10 to 20 L are generally used.
Most patients studied have been hemodynamically stable throughout the entire lavage procedure. In particular, lavage itself has caused no significant changes in systemic and transmural pulmonary artery pressure and cardiac output. [570] In these patients, arterial saturation, as measured by pulse oximetry, has increased and decreased with each lung filling and drainage, respectively.[570] In addition, cardiac output has decreased and increased with each lung filling and drainage, respectively. Arterial saturation increases during lung filling because blood flow to the nonventilated lung is decreased by the infusion pressure of lavage fluid.[571] The opposite set of events (increased blood flow in the nonventilated lung, decreased arterial oxygen saturation) occurs during lung drainage.[571] Not surprisingly, cardiac output decreases with each instillation of lavage fluid because of the increase in PVR.[570] In addition, with drainage of the lavage fluid, PVR decreases and cardiac output increases.[570]
If a small leak should occur during lavage, the following may be observed sequentially: (1) the appearance of bubbles in lavage fluid draining from the lavaged lung, (2) rales and rhonchi in the ventilated lung, (3) a difference between lavage volumes administered and those drained from the lavaged lung (the former exceeds the latter), and (4) a fall in arterial oxygen saturation. If a small leak is suspected or detected by any of these signs and the lavaged lung has been only minimally treated, the lavaged lung should be drained of all fluid, and the position of the DLT, the adequacy of cuff seal, and separation of the lungs should be rechecked with a fiberoptic bronchoscope. Before beginning the lavage procedure again and no matter what the DLT malposition was, the functional separation of the two lungs and cuff seal adequacy should be tested and found satisfactory by using the previously described air bubble leak detection method.
Massive spillage of fluid from the lavaged lung to the ventilated lung is not a subtle event and results in a dramatic decrease in ventilated lung compliance and a rapid and profound decrease in arterial oxygen saturation. Under these circumstances, the lavage procedure must be terminated no matter how much treatment has been accomplished. The patient should be moved quickly to the LDP with the lavaged side dependent, and the operating room table should be placed in a head-down position to facilitate the removal of lavage fluid. Vigorous suctioning and inflation of both lungs should be carried out. The DLT should be changed to a standard single-lumen tube, and the patient should additionally receive a period of mechanical ventilatory support with PEEP. Timing of further unilateral lung lavage attempts will be dictated by the patient's subsequent clinical course and gas exchange status.
After the effluent lavage fluid becomes clear, the procedure is terminated. The lavaged lung is thoroughly suctioned, and ventilation to that lung is resumed. Because the compliance of the lavaged side will be much less than that of the ventilated side at this time, large tidal ventilations (sighs) (15 to 20 mL/kg) to that side alone (with the nonlavaged side temporarily nonventilated) are necessary to reexpand the alveoli. Arterial blood oxygenation may decrease precipitously during this time, but this decreased oxygenation can be minimized by clamping the nonlavaged side after a large inspiration of 100% oxygen.
After lavage, the recovery procedure consists of repetitive periods
of large tidal ventilations, suctioning, and chest wall percussion to the previously
lavaged side; conventional two-lung ventilation with PEEP; and bilateral suctioning
and postural drainage while intermittently measuring combined (total) and individual
lung dynamic compliance. As the compliance of the lavaged lung returns to prelavage
values, ventilation with an air-oxygen mixture may help lavaged lung alveoli with
low V̇/![]() ratios to remain open. When the compliance of the hemothorax of
the lavaged side returns to its prelavage value, the neuromuscular blockade is reversed.
Mechanical ventilation and extubation guidelines are the same as for any patient
with pulmonary disease; most patients are able to be extubated while still in the
operating room.
ratios to remain open. When the compliance of the hemothorax of
the lavaged side returns to its prelavage value, the neuromuscular blockade is reversed.
Mechanical ventilation and extubation guidelines are the same as for any patient
with pulmonary disease; most patients are able to be extubated while still in the
operating room.
In the immediate postlavage period, deep breathing (incentive spirometry), coughing exercises, chest percussion, and postural drainage are used to remove the remaining fluid and secretions and to reexpand the lavaged lung further. After 3 to 5 days of recovery, the patient is returned to the operating room to have the opposite side lavaged. Anesthetic considerations for the second lavage are the same as for the first lavage, although oxygenation is not usually nearly as severe a problem as during the first lavage because the treated and now near-normal lung will be used to support gas exchange.
Special problems associated with pulmonary lavage that may be encountered are that a few very critically ill patients may be unable to tolerate the conventional procedure and unilateral lavage through a DLT is not easily done in children. Alternative (and more complicated) ways of accomplishing lung lavage in these patients include the use of extracorporeal membrane oxygenation and lobar lavage through a fiberoptic bronchoscope inserted under topical anesthesia.[260]
At the confluence of the superior, anterior, and middle mediastinum are the middle portion of the superior vena cava, the tracheal bifurcation, the main pulmonary artery, the aortic arch, and parts of the cephalad surface of the heart. [260] In adults, most tumors in this region originate from involvement of the hilar lymph nodes with bronchial carcinoma or lymphoma, whereas in babies, the masses are most often benign bronchial cysts, esophageal duplication, or teratoma. Tumors in this region can cause compression and obstruction of three of the vital mediastinal structures: the tracheobronchial tree in the region of the tracheal carina, the main pulmonary artery and atria, and the superior vena cava. A CT scan of the chest is probably the single most important diagnostic procedure because it defines the size and degree of compression of these vital structures. The most common complication that occurs during anesthesia for masses involving these three vital mediastinal structures is airway obstruction, which occurred in 20 of 22 patients in one review.[572] Although airway obstruction has been predominant in terms of symptomatology, it is not uncommon for compression of two or three of the major organs to be present in varying degrees and to cause complications in the same patient.[572] Each of these complications is life threatening and can cause acute deterioration and death during anesthesia if not handled with the most extreme caution and expertise. Each major complication and anesthetic management problem is discussed separately in the following sections, and Figure 49-39 shows the overall strategy for managing these three problems.
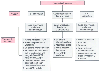 Figure 49-39
Flow diagram showing the essential management strategies
for the three major anesthetic problems that mediastinal masses may cause. CPB,
cardiopulmonary bypass; ET T, endotracheal tube; FOB, fiberoptic bronchoscopy; PA,
pulmonary artery; SVC, superior vena cava; TBT, tracheobronchial tree. (From
Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Figure 49-39
Flow diagram showing the essential management strategies
for the three major anesthetic problems that mediastinal masses may cause. CPB,
cardiopulmonary bypass; ET T, endotracheal tube; FOB, fiberoptic bronchoscopy; PA,
pulmonary artery; SVC, superior vena cava; TBT, tracheobronchial tree. (From
Benumof JL: Anesthesia for Thoracic Surgery. Philadelphia, WB Saunders, 1987.)
Most anterior mediastinal masses that cause airway obstruction [260] are lymphomatous in origin. However, a number of benign conditions such as cystic hygroma, teratoma, thymoma, and thyroid tumors can occur in a similar fashion. A tissue diagnosis should therefore be made before radiotherapy or chemotherapy is undertaken. Thus, most patients with a mediastinal mass causing airway obstruction will first require anesthesia for a diagnostic procedure (e.g., cervical or scalene node biopsy, staging laparotomy for Hodgkin's disease). Importantly, not all patients in whom severe intraoperative respiratory problems develop have respiratory symptoms and signs preoperatively.
Anesthetic management of these patients is based on two overriding considerations. First, obstruction of major airways by a tumor is usually life threatening because the obstruction generally occurs around the bifurcation of the tracheobronchial tree and is therefore distal to the endotracheal tube. The loss of spontaneous ventilation seems to precipitate the airway obstruction. It may be that loss of chest wall tone and the distending forces of active inspiration after the administration of muscle relaxants release the extrinsic support of a critically narrowed airway. Alternatively, intubation in the presence of distortion or compression of the trachea may cause complete obstruction if the orifice of the tube impinges on the tracheal wall or if the lumen of the tube is occluded where it passes a narrowed section or turns a sharp angle. In view of the potential for fatal airway obstruction during general anesthesia, all possible attempts to perform the procedure under local anesthesia should be undertaken.
Second, the response of lymphomatous tumors to irradiation or chemotherapy is normally dramatic. Chest radiographs reveal a marked decrease in tumor size, and the symptoms are usually improved. Consequently, it behooves the treating physician to use radiotherapy or chemotherapy if at all possible (sometimes the cell type can be known with a reasonable degree of certainty without a biopsy) before general anesthesia is undertaken.[573] [574] It should be noted that during irradiation, a small window can be created to spare some tissue for adequate histologic diagnosis.[575] [576]
The following management plan is based on the principles outlined
in Table 49-22
(see Fig.
49-39
).[260]
[572]
[573]
[574]
[575]
[576]
| Perform all procedures under local anesthesia if possible. |
| Use radiotherapy and/or chemotherapy if possible before general anesthesia. |
| If general anesthesia is required, consider inspection of the tracheobronchial tree with a fiberoptic bronchoscope and intubate the patient while awake. |
| If general anesthesia is required, maintain spontaneous ventilation. |
If the patient does not have dyspnea and is tolerant of the supine position (i.e., is asymptomatic), a CT scan, flow-volume loop, and echocardiogram should be obtained to evaluate the anatomic and functional position of the tumor. If any of these three tests have positive results, local anesthesia should be used for biopsy even if the patient is asymptomatic.
If general anesthesia is to be used, the airway should be evaluated by fiberoptic bronchoscopy with topical anesthesia before induction of general anesthesia. [576] The fiberoptic bronchoscope should be jacketed with an armored endotracheal tube, and after the fiberoptic bronchoscopy examination has been completed, the patient is intubated. General anesthesia should be induced with the patient in the semi-Fowler position. The patient should be allowed to breathe spontaneously throughout the procedure; muscle relaxants should be avoided. Large changes in intrathoracic pressure, which may promote collapse of a weakened tracheobronchial tree, must be prevented. The operating room team should retain the capability of changing the patient's position rapidly to the lateral or prone position. A rigid ventilating bronchoscope should be on hand to bypass distal tracheal and carinal obstructions, and the appropriate personnel and equipment for cardiopulmonary bypass should also be available.
These patients must be watched extremely closely in the first few postoperative hours. Airway obstruction requiring reintubation and mechanical ventilation has occurred, possibly secondary to an increase in tumor size as a result of tumor edema after instrumentation.
Compression of the pulmonary artery and heart is very rare because the pulmonary trunk is more or less protected by the aortic arch and tracheobronchial tree; only a few case reports of this problem have appeared in the literature.[260]
Principles similar to those for compression of the tracheobronchial tree apply to compression of the pulmonary artery. Most patients have their first anesthetic experience because they require a diagnostic procedure (e.g., a biopsy). These patients should be evaluated preoperatively in a manner similar to that used for patients with compression of the tracheobronchial tree. If the cell type is known or is highly suspected, preoperative irradiation should be seriously considered. All diagnostic procedures should be performed under local anesthesia if at all possible. If general anesthesia is required and if the symptoms worsen in the supine position, the sitting, leaning-forward, or even face-down position is advised, and spontaneous ventilation should be maintained throughout the procedure. Measures to maintain venous return, pulmonary artery pressure, and cardiac output, such as volume loading and the use of ketamine, should be considered. Arrangements for extracorporeal oxygenation should be completed preoperatively.
The superior vena cava syndrome is caused by mechanical obstruction of the superior vena cava. Causes of superior vena cava obstruction, in order of decreasing incidence, are bronchial carcinoma (87%), malignant lymphoma (10%), and benign causes (3%) such as central venous hyperalimentation and pacemaker catheter-induced thrombosis of the superior vena cava, idiopathic mediastinal fibrosis, mediastinal granuloma, and multinodular goiter.[260] The classic features of superior vena cava syndrome include dilated distended veins in the upper half of the body as a result of increased peripheral venous pressure (which can be as high as 40 mm Hg); edema of the head, neck, and upper extremities; dilated venous collateral channels in the chest wall; and cyanosis. Venous distention is most prominent in the recumbent position, but in most instances the veins do not collapse in the normal manner with the patient upright. Edema can be so great that it causes swelling of the periorbital fissures and thereby prevents patients from opening their eyes. Such advanced cases of edema usually obscure the dilated veins as well. Most patients have respiratory symptoms (shortness of breath, cough, orthopnea) that are due to obstruction of the airways by engorged veins and mucosal edema, and these are ominous signs. Similarly, a change in mentation as a result of cerebral venous hypertension and edema is a sign of particularly significant obstruction. In some cases, the superior vena cava becomes occluded quite slowly, and the signs and symptoms may be insidious in onset. When the occlusion occurs relatively rapidly, all clinical manifestations are more prominent. The most common radiologic sign is widening of the superior mediastinum. Venography confirms the diagnosis (but not the cause). Determination of etiology may require thoracotomy, sternotomy, bronchoscopy, lymph node biopsy, and other measures.
Most patients with superior vena cava syndrome caused by a malignant process are treated with irradiation and chemotherapy (for patients with incomplete obstruction).[577] However, in patients with nearly complete to complete obstruction (who usually have signs of cerebral venous hypertension or airway obstruction, or both) or in whom irradiation or chemotherapy proves ineffective, surgical bypass or resection of the lesion through a median sternotomy is indicated. [577] These operations are usually technically quite difficult because the tissue planes are poorly delineated, the anatomy is grossly distorted, central venous pressure is abnormally high, and varying degrees of fibrosis are present.
Preoperative anesthetic evaluation of a patient for superior vena cava decompression should include careful assessment of the airway. The same degree of edema that is present externally in the face and neck can be expected to be present in the mouth, oropharynx, and hypopharynx. In addition, the airway may be compromised by external
The patient is transported to the operating room in the head-up position to minimize airway edema. A radial artery catheter is inserted in all patients, and depending on the medical condition of the patient, a central venous or pulmonary artery catheter is inserted through the femoral vein before induction of anesthesia. At least one large-bore intravenous cannula should be inserted into the leg or femoral vein before operating. Premedication is best limited to an antisialagogue to reduce airway secretions. The method chosen for induction of anesthesia and intubation depends on the preoperative airway evaluation. If it is necessary for the patient to maintain the sitting position to achieve adequate ventilation before induction, intubation with the patient awake may be facilitated by using a fiberoptic laryngoscope or bronchoscope.
The most significant intraoperative problem encountered is bleeding. Substantial venous blood loss results from the abnormally high central venous pressure. Further, unexpected arterial bleeding may occur because of the difficulty of dissection in a distorted surgical field. Crossmatched blood should therefore be available in the operating room at the time of sternotomy.
Postoperatively, especially after diagnostic procedures such as mediastinoscopy and bronchoscopy wherein the superior vena cava obstruction has not been relieved, acute severe respiratory failure requiring intubation and mechanical ventilation may occur.[260] The mechanisms of the acute respiratory failure are obscure, but the most likely ones that are unique to superior vena cava syndrome are acute laryngospasm and acute bronchospasm (both caused by continued and perhaps increased obstruction of the superior vena cava), impaired respiratory muscle function (patients with malignant disease may have an abnormal response to muscle relaxants), and increased airway obstruction by the tumor (because of tumor swelling). Consequently, these patients must be closely monitored in the first few postoperative hours.
|
|
|
|
|
|
|
|
|
|
|
|
|