Diffusion Hypoxia
The uptake of large volumes of nitrous oxide on induction of anesthesia
gives rise to the concentration effect and the second gas effect. On recovery from
anesthesia, the outpouring of large volumes of nitrous oxide can produce what Fink
[96]
called diffusion anoxia.
These volumes may cause hypoxia ( Fig.
5-23
) in two ways. First, they may directly affect oxygenation by displacing
oxygen.[96]
[97]
[98]
Second, by diluting alveolar carbon dioxide,
they may decrease respiratory drive and ventilation.[98]
Both of these effects require the release of large volumes of nitrous oxide into
the alveoli. Because large volumes of nitrous oxide are released only during the
first 5 to 10 minutes of recovery, this is the period of greatest concern. The concern
is enhanced by the fact that the first 5 to 10 minutes of recovery also may be the
time of greatest respiratory depression. For these reasons, many anesthetists administer
100% oxygen for the first 5 to 10 minutes of recovery. This procedure may be particularly
indicated in patients with preexisting lung disease or in those in whom postoperative
respiratory depression is anticipated (e.g., after a nitrous oxide-narcotic anesthetic).
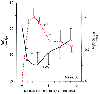 Figure 5-23
At time zero, the inspired gas was changed from 21% oxygen/79%
nitrous oxide to 21% oxygen/79% nitrogen. Arterial oxygen subsequently fell in association
with the outpouring of nitrous oxide. (Adapted from Sheffer L, Steffenson
JL, Birch AA: Nitrous oxide-induced diffusion hypoxia in patients breathing spontaneously.
Anesthesiology 37:436–439, 1972.)
Figure 5-23
At time zero, the inspired gas was changed from 21% oxygen/79%
nitrous oxide to 21% oxygen/79% nitrogen. Arterial oxygen subsequently fell in association
with the outpouring of nitrous oxide. (Adapted from Sheffer L, Steffenson
JL, Birch AA: Nitrous oxide-induced diffusion hypoxia in patients breathing spontaneously.
Anesthesiology 37:436–439, 1972.)
 Figure 5-23
At time zero, the inspired gas was changed from 21% oxygen/79%
nitrous oxide to 21% oxygen/79% nitrogen. Arterial oxygen subsequently fell in association
with the outpouring of nitrous oxide. (Adapted from Sheffer L, Steffenson
JL, Birch AA: Nitrous oxide-induced diffusion hypoxia in patients breathing spontaneously.
Anesthesiology 37:436–439, 1972.)
Figure 5-23
At time zero, the inspired gas was changed from 21% oxygen/79%
nitrous oxide to 21% oxygen/79% nitrogen. Arterial oxygen subsequently fell in association
with the outpouring of nitrous oxide. (Adapted from Sheffer L, Steffenson
JL, Birch AA: Nitrous oxide-induced diffusion hypoxia in patients breathing spontaneously.
Anesthesiology 37:436–439, 1972.)