|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RBCs are transfused primarily to increase transport of oxygen to tissues. An increase in the circulating red cell mass produces an increase in oxygen uptake in the lungs and a corresponding probable increase in oxygen delivery to tissues. The respiratory function of red cells may be impaired during preservation, making it difficult for them to release oxygen to the tissues immediately after transfusion.
In 1954, Valtis and Kennedy[51] first described a leftward shift of the oxygen dissociation curve in vitro, the magnitude of which was directly related to the length of time the acid citrate dextrose (ACD) blood had been stored. After transfusion of 7-day or older ACD blood, the oxygen dissociation curves of all patients also shifted to the left. The magnitude of the left shift was related to the volume and storage time of the infused ACD blood. In some cases, the curve remained shifted to the left for as long as 24 hours after transfusion.
The oxygen dissociation curve is determined by plotting the partial pressure of oxygen (PO2 ) in blood against the percentage of hemoglobin saturated with oxygen ( Fig. 47-2 ). As hemoglobin becomes more saturated, the affinity of
 Figure 47-2
Factors that shift the oxygen dissociation curve. 2,3-DPG,
2,3-diphosphoglycerate. (From Miller RD: The oxygen dissociation curve
and multiple transfusions of ACD blood. In Howland
WS, Schweizer O (eds): Management of Patients for Radical Cancer Surgery. Clinical
Anesthesia Series, vol 9. Philadelphia, FA Davis, 1972, p 43.)
Figure 47-2
Factors that shift the oxygen dissociation curve. 2,3-DPG,
2,3-diphosphoglycerate. (From Miller RD: The oxygen dissociation curve
and multiple transfusions of ACD blood. In Howland
WS, Schweizer O (eds): Management of Patients for Radical Cancer Surgery. Clinical
Anesthesia Series, vol 9. Philadelphia, FA Davis, 1972, p 43.)
Shifts in the oxygen dissociation curve are quantitated by the P50 , which is the partial pressure of oxygen at which hemoglobin is half saturated with oxygen at 37°C and pH 7.4. A low P50 indicates a left shift in the oxygen-dissociation curve and an increased affinity of hemoglobin for oxygen; in other words, the left shift of the curve indicates that a lower-than-normal oxygen tension saturates hemoglobin in the lung and the subsequent release of oxygen to the tissues occurs at a lower-than-normal capillary oxygen tension. An increased affinity may be enough to ensure that oxygen is released to the tissues unless the tissue PO2 is in the hypoxic range. The clinical evidence supporting the accuracy of this hypothesis during infusion is discussed in the following section.
The clinical evidence is not consistent, reflecting the difficulty of conducting a systematic study of seriously ill patients in varied clinical settings. Kopriva and colleagues[52] found that 2,3-DPG levels decreased in 31 seriously injured battle casualties, each of whom received 12 or more
although this evidence is suggestive, specific organ hypoxia has not been shown to result from infusion of blood with a low P50 or from increased affinity for oxygen. Valeri and Collins[55] performed a study in a group of patients who especially depended on adequate levels of 2,3-DPG for oxygen transport. Patients who had anemic hypoxia were transfused with three to five units of washed, liquid-stored RBCs that were depleted of 2,3-DPG and experienced an increased affinity for oxygen. No change in cardiac index or oxygen consumption resulted. Even in patients in whom 2,3-DPG is especially important for oxygen transport, no changes occurred. Bowen and Fleming[56] showed that although oxyhemoglobin affinity increases after transfusion of stored blood, arteriovenous oxygen extraction by organs or tissue might not be altered by changes in oxyhemoglobin affinity, particularly if a compensatory flow mechanism takes place at the capillary level. Such mechanisms may open capillaries, permitting increased blood flow to tissue, thereby increasing cardiac output and reducing the capillary tissue oxygen gradient to maintain the rate of tissue oxygen extraction. Because of the low P50 and the increased oxyhemoglobin affinity of stored blood, assessment of specific organ function is necessary to substantiate the possible injurious effect of stored blood. In 1993, Marik and Sibbard[8] found that the administration of blood that had been stored for more than 15 days actually decreased intramucosal pH, suggesting that splanchnic ischemia had occurred. In the past 10 years, numerous studies have tried to prove that older blood (and therefore decreased oxygen delivery) is not as beneficial as fresher blood in critically ill patients. In the "Storage of Blood" section, references [49] and [50] are examples of such studies. Although a definitive conclusion cannot be made, I believe that blood with less than 15 days of storage should be used in very ill patients.
Unless a patient has a preoperative coagulopathy (e.g., aspirin, antiplatelet drugs, hemophilia), a transfusion-induced coagulopathy usually occurs only after a large amount of blood has been given (e.g., 6 to 10 units of PRBCs). Various protocols have been developed for approaches to massive blood transfusion administration ( Fig. 47-3 ). This coagulopathy is caused by a combination of factors, of which the most important are the volume of blood given and the duration of hypotension or hypoperfusion.[57] Patients who are well perfused and are not hypotensive for a long period (e.g., 1 hour) can tolerate multiple units of blood without developing a coagulopathy. The patient who is hypotensive and has received many units of blood probably has a coagulopathy from a condition that resembles disseminated intravascular coagulation (DIC) and dilution of coagulation factors from stored bank blood. When such bleeding occurs, the differential diagnosis for a patient who did not have a pretransfusion coagulopathy (e.g., hemophilia) is dilutional thrombocytopenia, low factors V and VIII, a DIC-like syndrome, or hemolytic transfusion reaction. Clinical manifestations include oozing into the surgical field, hematuria, gingival bleeding, petechial bleeding from venipuncture sites, and ecchymoses.
Dilutional thrombocytopenia is a cause of a hemorrhagic diathesis in a patient who has received multiple units of bank blood Independent of whether whole blood or PRBCs are given, few viable platelets exist when storage has been for more than 24 hours. For whole blood at a storage temperature of 4°C, platelets in stored blood are damaged sufficiently to be readily trapped and absorbed by the reticuloendothelial system soon after infusion. Even those platelets that are not immediately stored have a reduced survival time. Considering survival time and viability, total platelet activity is only 50% to 70% of the original in vivo activity after 6 hours of storage in bank blood at 4°C. After 24 or 48 hours of storage, platelet activity is only about 10% or 5% of normal, respectively. Infusion of bank blood stored for longer than 24 hours dilutes the available platelet pool. In my study during the Vietnam conflict, platelet counts decreased to less than 100,000/mm3 when 10 to 15 units of blood had been given to acutely wounded, previously healthy soldiers.[58] The platelet count in smaller, older patients may decrease to 100,000/mm3 after fewer units of blood because these patients have a smaller blood volume and possibly a lower preoperative platelet count than soldiers. Miller and coworkers[58] defined the importance of the platelet count, because when it is approximately 75,000/mm3 or less, a hemorrhagic diathesis is likely to occur ( Table 47-5 ).
Although major emphasis had been placed on monitoring the platelet count, several investigators[57] [59] [60] have questioned the role of dilutional thrombocytopenia in the coagulopathy of massively transfused patients. They correctly point out that the platelet count rarely decreases to as low a level as would be predicted from dilution alone ( Fig. 47-4 ). This is probably because platelets are released into the circulation from the spleen and bone marrow and because of the presence of nonfunctional platelets. Reed and associates[60] found no benefit to prophylactic platelet administration during massive transfusion. Platelets should not be given to treat laboratory evidence of thrombocytopenia unless clinical coagulopathy is also present. Treating laboratory numbers without correlation with the clinical status is fundamentally contrary to good medical practice; transfusion medicine is no exception. When the platelet count is less than 50,000 to 75,000/mm3 , a bleeding problem is likely and is probably a combination of dilutional thrombocytopenia and DIC. Platelet therapy would be appropriate in this situation (see "Platelet Concentrates").
 Figure 47-3
This algorithm for diagnosing and treating a massive
transfusion was modified from the massive transfusion protocol used at the San Francisco
General Hospital. There are many other similar approaches; Figure
47-7
provides an algorithm for a pure coagulation. This protocol suggests
how to approach a patient with major blood loss but does not include the use of recombinant
activated factor VII, which may be possible in the future.[75]
BP, blood pressure; CBC, complete blood cell count; EBV, effective blood volume;
ED, emergency department; FFP, fresh frozen plasma; Hct, hematocrit; INR, international
normalized ratio; PC, platelet count; PRBC; packed red blood cells; PT, prothrombin
time; PTT, partial thromboplastin time.
Figure 47-3
This algorithm for diagnosing and treating a massive
transfusion was modified from the massive transfusion protocol used at the San Francisco
General Hospital. There are many other similar approaches; Figure
47-7
provides an algorithm for a pure coagulation. This protocol suggests
how to approach a patient with major blood loss but does not include the use of recombinant
activated factor VII, which may be possible in the future.[75]
BP, blood pressure; CBC, complete blood cell count; EBV, effective blood volume;
ED, emergency department; FFP, fresh frozen plasma; Hct, hematocrit; INR, international
normalized ratio; PC, platelet count; PRBC; packed red blood cells; PT, prothrombin
time; PTT, partial thromboplastin time.
To place such emphasis on the platelet count as a guide is appropriate,
as defended previously, with some exceptions. For example, patients with chronic
thrombocytopenia or leukemia are commonly known to survive and not have a hemorrhagic
diathesis with a platelet count lower than 15,000 cells/mm3
. However,
this does not negate the general guideline that patients with a platelet count of
less than 75,000 cells/mm3
are likely to bleed. For unexplained reasons,
patients with an acutely induced thrombocytopenia (e.g., from blood transfusions)
develop a hemorrhagic diathesis at a much higher platelet count than do patients
with a chronically induced thrombocytopenia (e.g., idiopathic thrombocytopenia purpura).
A higher platelet count is required to maintain adequate hemostasis with a surgical
incision or trauma, because damaged capillaries require platelets to plug the holes.
| Platelet Count (cells/mm3 ) | Total No. of Patients | No. of Patients with Bleeding |
|---|---|---|
| >100,000 | 21 | 0 |
| 75,000–100,000 | 14 | 3 |
| 50,000–75,000 | 11 | 7 |
| <50,000 | 5 | 5 |
| Data from Miller RD, Robbins TO, Tong MJ, et al: Coagulation defects associated with massive blood transfusions. Ann Surg 174:794, 1971. | ||
 Figure 47-4
Mean platelet counts after massive transfusions in relation
to number of units of blood transfused. Observed versus predicted values calculated
on the basis of blood exchange model. (From Myllylä G: New transfusion
practice and haemostasis. Acta Anaesthesiol Scand Suppl 89:76, 1988.)
Figure 47-4
Mean platelet counts after massive transfusions in relation
to number of units of blood transfused. Observed versus predicted values calculated
on the basis of blood exchange model. (From Myllylä G: New transfusion
practice and haemostasis. Acta Anaesthesiol Scand Suppl 89:76, 1988.)
Most of the factors are stable in stored blood, with two exceptions: factors V and VIII.[61] These factors gradually decrease to 15% and 50% of normal, respectively, after 21 days of storage. Packed RBCs even have fewer coagulation factors. Consequently, administration of fresh frozen plasma (FFP), which contains all the factors except platelets, has been recommended on a therapeutic or a prophylactic basis. However, this practice is of questionable benefit because only 5% to 20% of factor V and 30% of factor VIII are needed for adequate hemostasis during surgery. In other words, in spite of a patient's receiving massive blood transfusion, factors V and VIII rarely decrease below those levels required for hemostasis. Miller and colleagues[58] examined this problem by giving 500 to 1000 mL of FFP to five patients who had received more than 15 units of bank blood and who had a clinically significant hemorrhagic diathesis. Despite the partial thromboplastin times (which measure all factors except VII and XIII) and platelets' having returned to normal, bleeding persisted in every patient. Only when platelets in the form of fresh blood were administered did bleeding cease.[58] Although low factors V and VIII appear to be an unlikely primary cause of bleeding during massive blood transfusion, such deficiencies may intensify bleeding from other causes, usually dilutional thrombocytopenia in the case of blood transfusion.
In 1985, the National Institutes of Health conducted a consensus conference on this issue.[62] The conference concluded that there was little or no scientific evidence for the administration of FFP as part of the therapy for coagulopathy induced by multiple blood transfusions. The following criteria should be used:
The coagulation system consists of clotting and fibrinolytic mechanisms. The function of the former is to prevent excessive blood loss, and that of the latter is to ensure circulation within the vasculature. With this DIC-like syndrome, the clotting system is deranged, and this leads to disseminated fibrin deposition, which renders the fluid blood unclottable. The deposited fibrin may severely alter the microcirculation and lead to ischemic necrosis in various organs, particularly the kidney. The unclottable blood or circulating serum may induce a severe hemorrhagic diathesis.
The specific reasons for the development of syndrome are usually not apparent. However, hypoxic acidotic tissues with stagnant blood flow probably release tissue thromboplastin directly or through liberation of some toxin. The release of tissue plasminogen activator from damaged tissue may cause fibrinolysis. In sepsis and eventual organ failure, the pathogenesis of this DIC syndrome is more apparent. The extrinsic route of coagulation is activated by tumor necrosis factor and endotoxins. Presumably, tumor necrosis factor induces tissue factor expression on the surface of activated monocytes and possibly by exposure to subendothelially localized tissue factor in blood.[63] Although the intrinsic system does not induce DIC, it may contribute to hypotension. This triggers the coagulation process, resulting in consumption of factors I, II, V, and VIII and platelets. Supposedly, thrombi and fibrin are deposited in the microcirculation of vital organs, interrupting their blood flow.
In an attempt to counteract the hypercoagulable state, the fibrinolytic system is activated to lyse the excessive fibrin almost simultaneously; this is called secondary fibrinolysis. Primary fibrinolysis is rare and refers to activation of the fibrinolytic system without concomitant DIC. With secondary fibrinolysis, activation of plasminogen to plasmin is a protective mechanism that tends to prevent further DIC. With fulminate DIC and subsequent rapid depletion of coagulation factors, plasmin is formed from plasminogen at a rapid rate. The resultant fibrinolysis caused by plasmin creates a paradoxical state. Fibrinolysis does protect against further DIC but may also contribute to the severity of the bleeding diathesis. Plasmin digests fibrinogen, further reducing the fibrinogen level. The digestion of fibrinogen results in the formation of fibrinsplit products in the serum; the presence of these products indicates fibrinolysis. While the fibrinolytic system is actively trying to counteract DIC in early stages, plasminogen activator activity and plasmin generation rapidly decline, leaving DIC to progress unopposed.[63] It is at this stage that severe morbidity and eventually mortality
That DIC is a primary cause of organ failure, as suggested earlier, is an attractive hypothesis, but it has been challenged. Attar and coworkers[64] observed probable DIC in 294 patients with shock but found no fibrin deposits in 52 of them examined at autopsy. Survival rates of patients who have DIC associated with hypovolemia or septic shock are not increased by the administration of heparin. [65] [66] Mant and King[67] provided an excellent evaluation of 47 patients with severe acute DIC, mostly resulting from shock, infections, trauma, hepatic disease, and malignancy. Routine treatment included aggressive therapy of the underlying diseases and administration of blood products and vitamin K when indicated. Of the 47 patients, 12 were treated with heparin; bleeding worsened in seven (58%), and DIC diminished in five (42%). A total of 35 patients did not receive heparin; the DIC diminished in 13 (37%), but overall, 30 patients (86%) died. The investigators felt death could not have been prevented by heparin therapy. Microvascular thrombi were not found in 25 patients examined at autopsy. On the basis of these findings, Mant and King[67] accurately summarized the current knowledge of DIC:
Fourrier and colleagues[68] confirmed the aforementioned statements and concluded that DIC is a strong predictor of death. These investigators found that measurements of antithrombin III, protein C, and protein S levels were consistent with sustained DIC and inhibition of fibrinolysis. They stated that initial antithrombin III levels were the best predictor of death in septic patients.
The appearance of a hemorrhagic diathesis after blood transfusion should signal the possibility of a hemolytic transfusion. This entity is discussed later in this chapter.
Although treatment is more likely to be successful when the cause of the bleeding problem has been identified, precise diagnosis is often difficult. The more common, readily available laboratory tests seldom yield information precise enough to establish an accurate diagnosis.[61] For example, thrombocytopenia is most likely on a dilutional basis but could be secondary to some DIC-like syndrome alone or DIC associated with a hemolytic transfusion reaction. Laboratory tests offering more precise information, such as euglobulin lysis time, often are not readily available or take too long to perform to be practical in an emergency situation.
When the problem of a clinical hemorrhagic diathesis associated with blood transfusions occurs, one approach is to obtain a blood specimen on which the following tests can be performed: platelet count, partial thromboplastin time, plasma fibrinogen level, and observation of a clot for size, stability, and lysis and of the plasma for evidence of hemolysis. For many years, thromboelastography and assessment of the viscoelastic properties of plasma (Sonoclot)[69] have occasionally been recommended for monitoring the influence of blood loss and transfusions on coagulation. However, these techniques have not been widely accepted.
Although many other diagnostic approaches probably are equally valid, the preceding approach works well for me. Provided that the partial thromboplastin time is 1.5 times normal or more increased and other tests are normal, the bleeding is probably a result of very low levels of factors V and VIII. This can be treated with FFP, which contains all the coagulation factors except platelets, or with cryoprecipitate. Although the preceding situation is a nice textbook description, I have never observed clinical situation involving blood transfusions in which the partial thromboplastin time was increased without the presence of thrombocytopenia.
Dilutional thrombocytopenia in association with DIC is the most likely cause of bleeding from blood transfusion.[58] [61] When the platelet count is less than 100,000/mm3 , a bleeding problem is likely to develop (see Table 47-4 ); therefore, platelets are ordered. Unfortunately, the common delay between ordering and receiving the platelets dictates that they are ordered before the appearance of a hemorrhagic diathesis. The rule of thumb is based on the fact that a bleeding diathesis probably will develop after infusion of 20 units of stored blood in healthy patients and after lesser amounts in debilitated or small patients ( Fig. 47-5 ). Platelets should be ordered after infusion of 9 or 10 units of blood when several more will probably be required. Ideally, the platelets are available when 20 to 25 units of blood have been administered. The timing for ordering platelets in relation to when they will actually be required depends on the capabilities and the limitations of the local blood bank, which differ widely throughout the United States. The anesthesiologist and the surgeon must consult the blood bank before the need for platelets or any other blood component emerges.
Whether platelets are administered in the form of fresh blood, platelet-rich plasma, or platelet concentrates depends on volume replacement requirements, personal preference, and availability of laboratory personnel. Fresh blood (<6 hours old) supplies the largest number of platelets per donation. More than 80% of the platelets can be given by platelet-rich plasma, which has one half of the volume of a unit of blood. However, because most
 Figure 47-5
Correlation between units of blood administered and percent
of patients who had a hemorrhagic diathesis. The numbers in parentheses represent
the number of patients at each data point. (From Miller RD: Transfusion
therapy and associated problems. Reg Refresher Course Anesthesiol 1:101, 1973.)
Figure 47-5
Correlation between units of blood administered and percent
of patients who had a hemorrhagic diathesis. The numbers in parentheses represent
the number of patients at each data point. (From Miller RD: Transfusion
therapy and associated problems. Reg Refresher Course Anesthesiol 1:101, 1973.)
Although logistically difficult to obtain, fresh blood has been found to be extremely effective in treating transfusion-induced coagulopathies. My personal and subjective observations in Vietnam indicated that fresh blood (i.e., 6 hours or less and unrefrigerated blood) had a dramatic effect in patients with extensive hemorrhage.[58] About 20 years later, Lavee and associates[70] found that 1 unit of fresh whole blood was as effective as, if not superior to, 8 to 10 platelet units. In 1996, Erber and colleagues,[71] used fresh unrefrigerated whole blood in surgical patients with ongoing extensive bleeding despite adequate component replacement therapy and adequate surgical hemostasis. An accompanying editorial expressed caution and described the unfortunate problems with conducting a larger trial with fresh blood.[72] I believe that fresh blood also contains unidentified factors that make it far more effective than blood components.
Determining the plasma fibrinogen level is useful because this coagulation factor does not decrease in bank blood. If the in vivo plasma fibrinogen level is low (<150 mg/100 mL), it is not a result of a dilutional coagulopathy and strongly suggests DIC or a DIC-like syndrome. DIC is likely with thrombocytopenia, hypofibrinogenemia, and lysis of a clot within 2 hours.[61] Unfortunately, fibrinogen levels in PRBCs decrease with increasing storage time. As a result, hypofibrinogenemia occurs on a dilutional basis when multiple units of PRBCs are given.[73] The separation of thrombocytopenia on a dilutional basis versus DIC cannot be accomplished by the use of fibrinogen level when PRBCs have been given. Perhaps more specific readily available tests will be available in the future,[68] which will be especially important with potential therapies that are currently unavailable (e.g., monoclonal antibodies, anti-tumor necrosis factor antibodies, recombinant interleukin/receptor antagonists).[63] As indicated previously, the most effective treatment of DIC is removal or treatment of the basic disease process causing the DIC; these diseases usually cause DIC by release of damaged tissue products into the circulation. For example, the DIC associated with abruptio placentae usually ceases after emptying of the uterus and restoration of blood volume.
epsilon-Aminocaproic acid (EACA) inhibits the formation of plasmin and attenuates fibrinolysis. EACA should not be used in the treatment of DIC. Blocking the fibrinolytic system and having the coagulation system activated have resulted in disseminated thrombosis. Because primary fibrinolysis is rare other than in prostatectomy and liver transplantation[74] (see Chapter 56 ), EACA should probably not be given unless the preceding diagnosis is clearly established after expert consultation. Despite all of the previous recommendations, bleeding from a transfusion-associated coagulopathy occasionally persists. A new approach as been described. Administration of recombinant activated coagulation factor VII (rFVIIa, Novo Nordisk Pharmaceuticals, Plantation, FL) has produced successful treatment of such coagulopathies intraoperatively. Most of these patients also had other conditions, such as necrotizing pancreatitis, cirrhosis, or severe trauma. This exciting product is extremely expensive and should be viewed as a rescue therapy until FDA approval is more broadly based.[75]
In addition to EACA, three other drugs have been recommended for perioperative coagulation problems. Two of those drugs have received special attention. The first is 1-deamino-8-D-arginine vasopressin (DDAVP), a synthetic analog of the antidiuretic hormone vasopressin. It increases the levels of factor VIII and von Willebrand factor and is therefore well-established therapy for hemophilia and von Willebrand disease. It also reduces blood loss and transfusion requirement in patients with normal preoperative coagulation status who are undergoing spinal or cardiac surgery. However, the ultimate role of DDAVP remains to be determined.[76] DDAVP can cause hypotension, hyponatremia, and increased platelet adhesion.
Another drug is aprotinin, a serine protease inhibitor that inhibits fibrinolysis and improves platelet function.[77] It has been used to decrease blood loss in multiple surgical procedures, including cardiopulmonary bypass. However, its ultimate place in the treatment of coagulopathies has not been established.
The third drug is tranexamic acid, which is also an antifibrinolytic drug. Two studies found a decreased blood loss from total-knee arthroplasty.[78] [79] Presumably, release of the pneumatic tourniquet releases fibrinolytic material, which is inhibited by tranexamic acid.
A large meta-analysis using perioperative blood transfusion as the outcome in cardiac surgery concluded that aprotinin and tranexamic acid, but not DDAVP, decreased the exposure of patients to allogeneic blood transfusion perioperatively. [80] The ultimate use of these drugs is still evolving.
Most studies have examined the influence of massive transfusion of whole blood on coagulation because many trauma centers use whole blood. However, PRBCs (see "Packed Red Blood Cells") are often given because whole blood may not be available. With much less plasma, dilution of certain coagulation values may be more profound with the use of PRBCs rather than whole blood.
Murray and coworkers[73] specifically examined the question of using PRBCs for major blood loss. In general, the direction of coagulation changes was similar to that seen with whole blood, with one major exception. With use of PRBCs, fibrinogen levels decreased significantly in contrast to use of whole blood, in which fibrinogen levels remained unchanged unless DIC was present ( Fig. 47-6 ). Although all the coagulation factors decreased, the decrease was less than expected from dilution. The researchers felt that factors such as VIII are probably stored in endothelial cells and released from the endothelium during surgical stress. When PRBCs are used to replace major blood loss, the clinician may be tempted to give FFP prophylactically. However, Murray and associates[73] specifically recommended not following the policy; they stated that FFP was needed only when prothrombin time and partial thromboplastin time were
 Figure 47-6
Decreases in fibrinogen level as blood volume is replaced
with Adsol-packed red blood cells and crystalloid solutions. Each patient is represented
by a solid line. (From Murray DJ, Olson J, Strauss R, Tinker JH: Coagulation
changes during packed red cell replacement of major blood loss. Anesthesiology 69:839,
1988.)
Figure 47-6
Decreases in fibrinogen level as blood volume is replaced
with Adsol-packed red blood cells and crystalloid solutions. Each patient is represented
by a solid line. (From Murray DJ, Olson J, Strauss R, Tinker JH: Coagulation
changes during packed red cell replacement of major blood loss. Anesthesiology 69:839,
1988.)
An algorithm for the evaluation and initial therapy of a patient with a suspected coagulopathy is given in Figure 47-7 ).
Citrate intoxication is not caused by the citrate ion per se; it occurs because citrate binds calcium. The signs of citrate intoxication are those of hypocalcemia: hypotension, narrow pulse pressure, and elevated intraventricular end-diastolic pressure and central venous pressure. However, if circulatory volume is reasonably well maintained, these cardiovascular changes do not occur unless ACD blood is given at a rate more rapid than 150 mL/70 kg/min, or about 1 unit of blood per 5 minutes in an average-sized adult. With newer preservatives that contain less citrate, intoxication is even less likely.
Decreased serum levels of ionized calcium do occur in low flow states, especially in out-of-hospital cardiac arrests.[82] These decreases have no predictable relationship to total plasma concentration. A possible conclusion is that if blood is given to such a critically ill patient, the serum ionized calcium levels may decrease even more. However, Drop and Laver [83] found that these abnormally low concentrations of ionized calcium were not readily corrected by intravenous administration of calcium salts in doses generally recommended (i.e., 1.0 g of calcium chloride). However, ionized calcium levels returned toward normal when hemodynamic status was improved by increasing the isoproterenol infusion rate. The beneficial effect of isoproterenol probably indicates that mobilization ionized calcium from body stores may be inadequate because of abnormal distribution of blood flow. Improvement of a patient's circulatory status (i.e., blood transfusion) may ultimately increase ionized calcium levels without calcium administration. If calcium is given to patients with low-output states, calcium chloride may have to be administered at rates as rapid as 1.5 mg/kg/min. Drop and Laver[83] recommended that this amount of calcium chloride not be given without close monitoring of serum ionized calcium levels, because a constant relationship with total serum calcium level is not established. With the absence of ionized calcium electrodes, the well-known inotropic stimulation may lead the clinician to administer calcium any time evidence of inadequate cardiac output is present, especially when multiple blood transfusions have been given.
Even in patients with low-output states, I believe that emphasis should be placed on correcting the underlying disorder (i.e., hypovolemia) and that calcium administration is rarely necessary.[84] The reason that serum ionized calcium levels rapidly return to normal immediately after cessation of the blood transfusion, probably is rapid citrate
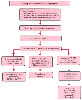 Figure 47-7
Algorithm of the evaluation and initial therapy of a
patient with suspected perioperative coagulopathy. The evaluation is based on the
clinical scenario and is affected by the type and location of injury, the amount
of fluid administered, and the age and body temperature of the patient. DDAVP, 1-deamino-8-D-arginine
vasopressin, a vasopressin analog also known as desmopressin acetate; PT, prothrombin
time; PTT, partial thromboplastin time. (Adapted from Habibi S, Corrsin
DB, McDermott JC, et al: Trauma and massive hemorrhage. In
Muravchick S, Miller RD (eds): Atlas of Anesthesia: Subspecialty Care. New York,
Churchill Livingstone, 1998, pp 6.2–6.17.)
Figure 47-7
Algorithm of the evaluation and initial therapy of a
patient with suspected perioperative coagulopathy. The evaluation is based on the
clinical scenario and is affected by the type and location of injury, the amount
of fluid administered, and the age and body temperature of the patient. DDAVP, 1-deamino-8-D-arginine
vasopressin, a vasopressin analog also known as desmopressin acetate; PT, prothrombin
time; PTT, partial thromboplastin time. (Adapted from Habibi S, Corrsin
DB, McDermott JC, et al: Trauma and massive hemorrhage. In
Muravchick S, Miller RD (eds): Atlas of Anesthesia: Subspecialty Care. New York,
Churchill Livingstone, 1998, pp 6.2–6.17.)
As evidenced from the preceding discussion, citrate intoxication is rare. Serum potassium levels may be as high as 19 to 30 mEq/L in blood stored for 21 days. Although hyperkalemia is occasionally reported,[85] large amounts of blood must be given. For significant hyperkalemia to occur clinically, bank blood must be given at a rate of 120 mL/min or more. The fact that such rapid infusion rates of blood are required for the production of hyperkalemia suggests that the potassium ion must leave the intravascular spaces by diffusion into extravascular spaces, by reuptake into RBCs, or through the kidneys. As with citrate intoxication, hyperkalemia is rare, and this also rules against the routine administration of calcium. Calcium may cause cardiac arrhythmias, particularly in patients anesthetized with halothane. Calcium administration should be based on diagnostic signs of hyperkalemia (i.e., peak T wave). Even though it is reported to be irritating to veins, 10% calcium chloride provides three times more calcium than an equal volume of 10% calcium gluconate because chloride has a molecular mass of 147 and gluconate a molecular mass of 448.
Administration of unwarmed blood that has been stored at 4°C can decrease the recipient's temperature. If the temperature decreases to less than 30°C, ventricular irritability and even cardiac arrest may occur. This can be prevented by warming the blood to body temperature before transfusion. I believe that there are more subtle reasons for warming all blood, even in patients receiving only 1 to 2 units intraoperatively. Because of the cool temperature of the operating room, body temperature often decreases, particularly in patients undergoing extensive abdominal surgery[86] (see Chapter 40 ); administration of cold blood further decreases temperature. A decrease in body temperature as small as 0.5°C to 1.0°C may induce shivering postoperatively; this may increase oxygen consumption by as much as 400%. To meet the demands of elevated oxygen consumption, cardiac output must be increased. Is this too much stress for the patient with marginal cardiac reserve? More studies are required to confirm this fear.
Perhaps the safest and most common method of warming blood is to pass it through plastic coils immersed in warm water (37° to 38°C) bath. With increased use of PRBCs (e.g., in contrast to whole blood), other methods of warming blood have been suggested. For example, Zorko and Polsky[87] added normal saline warmed to 45°C to PRBCs. Maximal flow rates are achieved with diluted cells not passed through a warmer, but delivery temperature is higher when passed through a warmer ( Table 47-6 ). Despite the aforementioned well-documented information, five cases of overheated hemolyzed blood have been reported to the FDA during the past 10 years. [88] A variety of warming techniques have been reviewed by Iserson and Heustis[89] (see Chapter 40 ).
The pH of most storage media is very acidotic (e.g., 5.5 for CPD).
When this solution is added to a unit of freshly drawn blood, the pH of the blood
immediately decreases to approximately 7.0 to 7.1. As a result of accumulation
| Status of Blood | Mean Flow Rate (g/min) | Delivered Temperature (°C) * |
|---|---|---|
| Undiluted and unwarmed | 11 | 18 |
| Undiluted and warmed † | 5 | 29 |
| Diluted ‡ and warmed | 18 | 35 |
| Diluted and unwarmed | 57 | 26 |
| From Zorko MF, Polsky SS: Rapid warming and infusion of packed red blood cells. Ann Emerg Med 15:907, 1986. | ||
 Figure 47-8
Correlation between the amount of blood administered
(mL) and corrected base excess intraoperatively. (From Miller RD, Tong MJ,
Robbins TO: Effects of massive transfusion of blood on acid-base balance. JAMA
216:1762, 1971.)
Figure 47-8
Correlation between the amount of blood administered
(mL) and corrected base excess intraoperatively. (From Miller RD, Tong MJ,
Robbins TO: Effects of massive transfusion of blood on acid-base balance. JAMA
216:1762, 1971.)
Although treatment of metabolic acidosis with bicarbonate has been viewed as being important, can any harm result from excessive bicarbonate administration or from metabolic alkalosis (see Chapter 41 and Chapter 78 ). Large doses of bicarbonate (e.g., 1 to 10 mEq/kg) can interfere with coagulation, as evidenced by prolonged prothrombin and thrombin clotting times.[93] Alkalosis augments a left shift of the oxygen dissociation curve. Because of citrate metabolism, exogenous bicarbonate, and administration of lactated Ringer's solution, metabolic alkalosis commonly occurs after infusion of several units of blood. Bicarbonate administration should be reserved for patients in whom severe metabolic acidosis (base excess > 7 mEq/L) has been diagnosed.
In 1970, Moseley and Doty[94] demonstrated that amounts of clot and debris in bank blood increased with duration of storage. Some of this particulate matter is not filtered by the standard 170-µm filter during routine transfusion and enters the recipient's blood stream.[94] These investigators suggest that respiratory insufficiency in patients with severe trauma and hemorrhage (so-called shock lung) or acute respiratory distress syndrome may be a result of the accumulation of this particulate material in the lungs, resulting in vascular obstruction. Several filters with pore sizes less than 40 µm (micropore filters) are now available to remove microaggregates from bank blood. When massive transfusions of stored blood are involved, the use of micropore filters, in theory, should eliminate this important contributor to the development of adult respiratory distress syndrome. However, the preceding concept and the need for micropore filters during massive transfusions of stored blood are unproved. The removal of white blood cells may reduce the infectivity of blood.[95] It is likely that prefiltered blood designed to remove most of the white blood cells will be routinely provided by blood banks in the future (see "Leukoreduction of Blood Transfusions"). Newer preservative solutions will probably eliminate microaggregates. The use of micropore filters continues to evolve.[96]
|
|
|
|
|
|
|
|
|
|
|
|
|