|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
There are many choices of drugs (see Chapter 12 ) in the United States to produce spinal anesthesia: procaine (novocain), lidocaine (Xylocaine), mepivacaine (Carbocaine), tetracaine (Pontocaine), ropivacaine (Neuropin), (S)-(-)-levobupivacaine (Chirocaine), and bupivacaine (Marcaine or Sensorcaine). These drugs provide spinal anesthetics that range from 45 to 400 minutes and offer two clinical lengths of action: shorter (<90 minutes) and longer (>90 minutes).
Procaine is one of the oldest spinal anesthetics and originally replaced cocaine as the drug of choice for spinal anesthesia early in the 20th century. It is used for brief spinal anesthetics (<1 hour) but appears to be used less frequently than lidocaine for these shorter spinal anesthetics because of three primary clinical differences. It is associated with a higher frequency of nausea (unexplained cause), a relatively high anesthetic failure rate, and a slower time to recovery. [64] It is often used as a hyperbaric drug in a dose ranging between 50 and 150 to 200 mg in a 10% concentration. The reason some continue in their use of procaine is the lower frequency of back and leg pain after its use compared with lidocaine.[65]
Lidocaine is also often chosen for shorter procedures that can be completed in 1.5 hours or less. It has measurable effect in less than 5 minutes and is most commonly used as the 5% solution in 7.5% dextrose; however, many
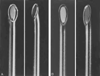 Figure 43-14
Continuous spinal needle examples, including a disposable,
18-gauge Hustead (A) and a 17-gauge Tuohy (B)
needle. Both have distal tips designed to direct the catheters inserted through
the needles along the course of the bevel opening; 20-gauge epidural catheters are
used with these particular needle sizes.
Figure 43-14
Continuous spinal needle examples, including a disposable,
18-gauge Hustead (A) and a 17-gauge Tuohy (B)
needle. Both have distal tips designed to direct the catheters inserted through
the needles along the course of the bevel opening; 20-gauge epidural catheters are
used with these particular needle sizes.
Mepivacaine is another drug useful for spinal anesthesia and is being used in settings in which lidocaine was used in the past. The drug-mass ratio for mepivacaine and lidocaine is approximately 1.3:1, suggesting mepivacaine can be used for spinal anesthesia in a 30- to 60-mg dose and typically in the 2% concentration. In many aspects, mepivacaine is a slightly longer-acting lidocaine, with variable reports of lower or equivalent rates of TNS compared with lidocaine.[71] [72] [73]
When longer-acting agents for spinal anesthesia are desired, four drugs are available: tetracaine, bupivacaine, ropivacaine, and levobupivacaine. Tetracaine has been used in spinal anesthesia for years and is packaged as niphanoid crystals (20 mg) and as a 1% solution (20 mg). This drug has an onset of 5 to 10 minutes and is selected for procedures lasting up to 2 to 3 hours when epinephrine is added and up to 5 hours for lower extremity procedures when phenylephrine (0.5 mg) is added as the vasoconstrictor.[74] [75] When niphanoid crystals are used, a 1% solution is mixed by adding 2 mL of sterile water (without preservatives) to the crystals. Then the appropriate milligram dose of 1% solution is mixed in equal volumes of 10% dextrose to produce a 0.5% tetracaine solution weighted with 5% dextrose. Subsequently, the vasoconstrictor may be added. Epinephrine and phenylephrine prolong spinal anesthesia with tetracaine ( Table 43-3 ).[77]
Bupivacaine spinal anesthesia is commonly carried out with 0.75%
and 0.5% solutions in dextrose, as well as
|
|
Duration of Blockade * | |
|---|---|---|
| Group | T10 (min) | L1 (min) |
| I: Tetracaine (plain) | 159 ± 41 | 230 ± 55 |
| II: Tetracaine + epinephrine (0.5 mg) | 234 ± 59 † | 327 ± 72 † |
| III: Tetracaine + phenylephrine (5 mg) | 273 ± 90 † | 406 ± 63 † ‡ |
| Adapted from Caldwell C, Nielsen C, Baltz T, et al: Comparison of high-dose epinephrine and phenylephrine in spinal anesthesia with tetracaine. Anesthesiology 62:804, 1985. | ||
Ropivacaine is an amide local anesthetic used frequently for epidural anesthesia because of experimental evidence of less effect than bupivacaine on the cardiac conduction system. With spinal anesthesia, this difference is minimal because small doses are used. Compared with bupivacaine, it is estimated to require 1.8 to 2 times the dose to produce a similar clinical effect.[82] [83] Many believe it is clinically indistinguishable from bupivacaine when the dose of drug administered is of a mass to produce an equal effect.[16]
Levobupivacaine is the isolated (S)-enantiomer of bupivacaine and available for use as a spinal anesthetic. Clinical data suggest this drug is bupivacaine when used for spinal anesthesia, and for doses ranging from 4 to 12 mg, a volunteer study suggests little clinical difference between levobupivacaine and racemic bupivacaine.[84] In 80 patients undergoing elective hip replacement and receiving isobaric levobupivacaine (3.5 mL of a 0.5% solution) or isobaric bupivacaine (3.5 mL of a 0.5% solution), the clinical efficacy was judged equivalent.[85] In a clinical situation in which systemic toxicity is a minimal issue (or the typical spinal anesthetic), the advantage of levobupivacaine over bupivacaine appears more theoretical than real.
Some physicians are concerned that the use of additives, particularly vasoconstrictors, may be risky. The concept is that epinephrine and phenylephrine have such potent vasoconstrictive action as to put the blood supply of the spinal cord at risk. There are no human data supporting this theory. Kozody and colleagues [76] [86] have shown that administering subarachnoid epinephrine (0.2 mg) or phenylephrine (5 mg) does not decrease spinal cord blood flow in dogs. These traditional vasoconstrictors are not the only adrenergomimetic agents being studied. Clonidine, an α2 -agonist, prolongs motor block associated with tetracaine spinal anesthesia in dogs as much as epinephrine while prolonging sensory blockade for an even longer interval.[87] The mechanism for this prolongation may involve vasoconstriction and antinociception from α-stimulation.[88] Another drug investigated for spinal use as an additive is neostigmine.[89] This acetylcholinesterase inhibitor inhibits the breakdown of acetylcholine that induces analgesia. It also prolongs and intensifies the analgesia through release of nitric oxide in the spinal cord. Despite the side effect of nausea and prolongation of motor block when combining it with local anesthetics, it is slowly gaining acceptance clinically.[90]
The interaction of various vasoconstrictors and local anesthetics is better understood. Traditionally, epinephrine was thought to prolong only tetracaine spinal anesthesia but not bupivacaine or lidocaine spinal anesthesia.[91] This theory was postulated because of differences in vasodilatory actions of the local anesthetic drugs; plain lidocaine and bupivacaine cause vasodilation, whereas plain tetracaine does not. The original investigations of spinal anesthetic duration used two-dermatome regression in the thoracic dermatomes to establish duration.[92] Since that time, it has become clearer that two-dermatome regression in the middle to high thoracic dermatomes may be misleading when measuring spinal anesthetic duration in the lower thoracic and lumbar dermatomes. There are data that lidocaine spinal anesthesia is prolonged by epinephrine when measured by two-dermatome regression in the lower thoracic dermatomes and by occurrence of pain at the operative site for procedures carried out at the level of lumbosacral dermatomes.[73] [93]
When epinephrine has been compared with phenylephrine as a means of prolonging spinal anesthesia, conflicting information has resulted. Concepcion and coworkers[94] compared epinephrine (0.2 and 0.3 mg) and phenylephrine (1 and 2 mg) added to tetracaine and did not find a difference in increased duration with the two vasoconstrictors. Caldwell and associates[77] used higher doses of vasoconstrictors, epinephrine at 0.5 mg and phenylephrine at 5 mg, and showed that phenylephrine prolonged tetracaine spinal anesthesia significantly more than epinephrine (see Table 43-3 ). Phenylephrine has been shown to prolong lidocaine spinal anesthesia, but it appears that the length of prolongation is more similar to that produced with epinephrine than significantly longer.[93] [95] Duration of bupivacaine spinal anesthesia does not appear to be prolonged by phenylephrine. [92] [96] Whichever local anesthetic solution and additives are selected for subarachnoid injection, special care should be taken to ensure that the clinician knows what substance is being injected and that all procedures have been carried out aseptically.
The density of any solution is the weight in grams of 1 mL of the solution at a standard temperature. Specific gravity is the density of a solution compared in a ratio with the density of water. Baricity is a ratio comparing the density of one solution to another. If the other solution happens to be water, the baricity will be the same as the specific gravity. To make a drug hypobaric to CSF, it must be less dense than CSF, having a baricity appreciably less than 1.0000 or a specific gravity appreciably less than 1.0069 (the mean value of CSF's specific gravity).
In the United States, a common method of formulating a hypobaric solution is to mix tetracaine in a 0.1% to 0.33% solution with sterile water. This makes the baricity of the solution less than 0.9977 and allows clinically useful anesthesia to be produced. In the prone position for anorectal procedures or in the lateral position for hip repairs, 4 to 6 mg of selected hypobaric dilution is often adequate. There is evidence that rate of injection (0.02 mL/sec versus 0.5 mL/sec) of hypobaric (0.2%) tetracaine influences spread of the drug.[97]
Another method of formulating a hypobaric-like solution is to use warmed 0.5% bupivacaine.[98] Data show that 0.5% bupivacaine warmed to 37°C, compared with 4°C, demonstrates hypobaric characteristics when the block is administered to sitting patients. The investigators
When isobaric spinal anesthesia is planned, the drugs most often chosen across the globe are bupivacaine, ropivacaine, and levobupivacaine (in 0.5% or 0.75% concentrations). Another drug that can be used is tetracaine. It is formulated into an isobaric solution by diluting the niphanoid tetracaine crystals (20 mg) with CSF and then injecting the selected mass of drug in an isobaric fashion.
|
|
|
|
|
|
|
|
|
|
|
|
|