Induction of Mild Therapeutic Hypothermia
Rapid core cooling may be required when the target core temperature
is low (e.g., 32°C or 33°C) or for stroke or acute myocardial infarction
when cooling must be induced expeditiously. In such cases, passive cooling is not
practical. Immersion in cold water is by far the quickest noninvasive method of
actively cooling patients. However, immersion is difficult under clinical conditions
and poses a substantial electrical safety risk. Administration of refrigerated intravenous
fluids is also effective and reduces mean body temperature 0.5°C/L.[183]
However, this method is usually impractical in neurosurgical patients, in whom fluids
must be restricted.
Forced-air cooling is easy to implement, but relatively slow,
with approximately 2.5 hours needed to cool neurosurgical patients to 33°C.[152]
Conventional circulating-water mattresses are unlikely to be efficient because relatively
little skin surface contacts the mattress and the patient's own body weight reduces
blood-borne convection of heat to the back. That is, circulating water probably
does not cool well for the same reason that it heats poorly.[139]
Newer circulating-water systems include garment-like covers that cover far more
skin surface and transfer large amounts of heat, and they are fairly effective.[177]
[184]
The best way to rapidly induce therapeutic hypothermia is probably
endovascular cooling. These systems consist of a heat-exchanging catheter, usually
inserted
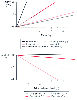 Figure 40-22
Relative effects of warming methods on mean body temperature
(ΔMBT) as a function of time (upper portion)
or administered fluid (lower portion). Mean body
temperature is the average temperature of body tissues and is usually somewhat less
than core temperature. The calculations assume an undressed 70-kg patient with a
metabolic rate of 80 kcal/hr, in thermal steady state, and with a typical 21°C
operating room environment. Only the changes resulting from specific treatments
are shown; changes from combined interventions will be additive. a to d, Changes
in MBT per liter of administered blood or crystalloid at various fluid temperatures;
e, inspiring warmed, humidified gas; f and g, warmed or unwarmed blankets, with all
skin below the neck covered; savings are similar with a single layer of other passive
insulators; h, full-length circulating water mattress; i, full-length forced-air
warmer. (Redrawn from Sessler AM, Sessler DI: Consequences and treatment
of perioperative hypothermia. Anesthiol Clin North Am 12:425–456, 1994. See
the original figure legend for equations and citations justifying the assumptions.)
Figure 40-22
Relative effects of warming methods on mean body temperature
(ΔMBT) as a function of time (upper portion)
or administered fluid (lower portion). Mean body
temperature is the average temperature of body tissues and is usually somewhat less
than core temperature. The calculations assume an undressed 70-kg patient with a
metabolic rate of 80 kcal/hr, in thermal steady state, and with a typical 21°C
operating room environment. Only the changes resulting from specific treatments
are shown; changes from combined interventions will be additive. a to d, Changes
in MBT per liter of administered blood or crystalloid at various fluid temperatures;
e, inspiring warmed, humidified gas; f and g, warmed or unwarmed blankets, with all
skin below the neck covered; savings are similar with a single layer of other passive
insulators; h, full-length circulating water mattress; i, full-length forced-air
warmer. (Redrawn from Sessler AM, Sessler DI: Consequences and treatment
of perioperative hypothermia. Anesthiol Clin North Am 12:425–456, 1994. See
the original figure legend for equations and citations justifying the assumptions.)
into the inferior vena cava through the femoral artery, and a servocontroller. They
can decrease core temperatures at rates approaching 4°C/hr ( Fig.
40-23
).[185]
Inducing therapeutic hypothermia during surgery is relatively
easy because anesthetics profoundly impair thermoregulatory responses. In contrast,
unanesthetized patients—even those who have suffered a stroke—vigorously
defend core temperature by vasoconstricting and shivering.[186]
It is thus necessary to pharmacologically induce tolerance to hypothermia. The
best method thus far identified is the combination of buspirone and meperidine, drugs
that synergistically reduce the shivering threshold to approximately 34°C without
provoking excessive sedation or respiratory toxicity.[187]
The combination of dexmedetomidine and meperidine may also be helpful, although
the interaction is simply additive in this case.
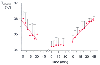 Figure 40-23
Average (± SD) intraoperative esophageal temperatures
(Tesoph
) during the cooling, temperature maintenance, and rewarming periods
in eight neurosurgical patients who were cooled with endovascular heat-exchanging
catheters in the vena cava. Time zero identified the beginning of each thermal management
period; the duration of these periods differed in individual patients, depending
on the length of surgery and other factors. The regression lines (gray
lines) are shown for the cooling and rewarming periods. (Redrawn
from Doufas AG, Akça O, Barry A, et al: Initial experience with a novel heat-exchanging
catheter in neurosurgical patients. Anesth Analg 95:1752–1756, 2002.)
Figure 40-23
Average (± SD) intraoperative esophageal temperatures
(Tesoph
) during the cooling, temperature maintenance, and rewarming periods
in eight neurosurgical patients who were cooled with endovascular heat-exchanging
catheters in the vena cava. Time zero identified the beginning of each thermal management
period; the duration of these periods differed in individual patients, depending
on the length of surgery and other factors. The regression lines (gray
lines) are shown for the cooling and rewarming periods. (Redrawn
from Doufas AG, Akça O, Barry A, et al: Initial experience with a novel heat-exchanging
catheter in neurosurgical patients. Anesth Analg 95:1752–1756, 2002.)
 Figure 40-22
Relative effects of warming methods on mean body temperature
(ΔMBT) as a function of time (upper portion)
or administered fluid (lower portion). Mean body
temperature is the average temperature of body tissues and is usually somewhat less
than core temperature. The calculations assume an undressed 70-kg patient with a
metabolic rate of 80 kcal/hr, in thermal steady state, and with a typical 21°C
operating room environment. Only the changes resulting from specific treatments
are shown; changes from combined interventions will be additive. a to d, Changes
in MBT per liter of administered blood or crystalloid at various fluid temperatures;
e, inspiring warmed, humidified gas; f and g, warmed or unwarmed blankets, with all
skin below the neck covered; savings are similar with a single layer of other passive
insulators; h, full-length circulating water mattress; i, full-length forced-air
warmer. (Redrawn from Sessler AM, Sessler DI: Consequences and treatment
of perioperative hypothermia. Anesthiol Clin North Am 12:425–456, 1994. See
the original figure legend for equations and citations justifying the assumptions.)
Figure 40-22
Relative effects of warming methods on mean body temperature
(ΔMBT) as a function of time (upper portion)
or administered fluid (lower portion). Mean body
temperature is the average temperature of body tissues and is usually somewhat less
than core temperature. The calculations assume an undressed 70-kg patient with a
metabolic rate of 80 kcal/hr, in thermal steady state, and with a typical 21°C
operating room environment. Only the changes resulting from specific treatments
are shown; changes from combined interventions will be additive. a to d, Changes
in MBT per liter of administered blood or crystalloid at various fluid temperatures;
e, inspiring warmed, humidified gas; f and g, warmed or unwarmed blankets, with all
skin below the neck covered; savings are similar with a single layer of other passive
insulators; h, full-length circulating water mattress; i, full-length forced-air
warmer. (Redrawn from Sessler AM, Sessler DI: Consequences and treatment
of perioperative hypothermia. Anesthiol Clin North Am 12:425–456, 1994. See
the original figure legend for equations and citations justifying the assumptions.) Figure 40-23
Average (± SD) intraoperative esophageal temperatures
(Tesoph
) during the cooling, temperature maintenance, and rewarming periods
in eight neurosurgical patients who were cooled with endovascular heat-exchanging
catheters in the vena cava. Time zero identified the beginning of each thermal management
period; the duration of these periods differed in individual patients, depending
on the length of surgery and other factors. The regression lines (gray
lines) are shown for the cooling and rewarming periods. (Redrawn
from Doufas AG, Akça O, Barry A, et al: Initial experience with a novel heat-exchanging
catheter in neurosurgical patients. Anesth Analg 95:1752–1756, 2002.)
Figure 40-23
Average (± SD) intraoperative esophageal temperatures
(Tesoph
) during the cooling, temperature maintenance, and rewarming periods
in eight neurosurgical patients who were cooled with endovascular heat-exchanging
catheters in the vena cava. Time zero identified the beginning of each thermal management
period; the duration of these periods differed in individual patients, depending
on the length of surgery and other factors. The regression lines (gray
lines) are shown for the cooling and rewarming periods. (Redrawn
from Doufas AG, Akça O, Barry A, et al: Initial experience with a novel heat-exchanging
catheter in neurosurgical patients. Anesth Analg 95:1752–1756, 2002.)