|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Two major obstacles are encountered when attempting to study the influence of inhaled anesthetics on the purified protein ionophores involved in the membrane translocation of ions. First, the biochemical purification of such protein ionophores in adequate quantities is a difficult and time-consuming process. Second, even when these membrane proteins can be isolated, they need to be reincorporated into lipids for measurement of their functional ability to translocate ions. This reincorporation of the purified ionophore into a lipid membrane makes it difficult to distinguish whether an anesthetic affects ion flow through a direct action on the membrane protein or through an indirect action on surrounding lipids.
The binding of neurotransmitter alters membrane permeability of ligand-operated channels to specific ions. The muscle-type nicotinic acetylcholine receptor-ionophore complex is the only channel complex that has been isolated in large enough quantities to be studied at the biochemical level with anesthetics. [128] This protein complex is composed of five polypeptide subunits, with each subunit containing four hydrophobic amino acid domains spanning the membrane; the subunits form the wall of the membrane channel. Each subunit of the acetylcholine receptor binds halothane, as measured by a radioligand-binding assay after photoactivation of radioactive halothane.[132] Volatile halogenated anesthetics stabilize the acetylcholine receptor in a conformational form that binds agonists with high affinity and is associated with a desensitized and therefore inactive (closed-channel) state.[133] However, not all anesthetics produce this effect. The non-halogenated alkane anesthetics cyclopropane and butane do not potentiate agonist actions on the acetylcholine
Additional information concerning anesthetic action on the nicotinic acetylcholine receptor ionophore may be made by examining ion flow through channels of defined subunit composition incorporated (through cDNA or mRNA injection) into cellular systems.[50] [135] Such studies have revealed a markedly enhanced sensitivity of neuronal (compared with muscular) nicotinic acetylcholine receptors to inhaled anesthetics ( Fig. 4-13 ).[136] [137] Because neuronal nicotinic acetylcholine receptors can be inhibited at inhaled anesthetic concentrations as low as 0.1 MAC (see Fig. 4-13 ), these receptors may play some role in the behavioral and physiologic effects observed at subanesthetic concentrations.[36] [136] The ability of specific amino acid mutations between the second and third transmembrane segment of the α-subunit of the neuronal acetylcholine receptor to markedly influence halothane sensitivity suggests that this region of the acetylcholine receptor is important for the transduction of anesthetic binding to channel inhibition.[137]
Other ligand-gated ion channels, including the inhibitory GABAA and glycine channels ( Fig. 4-14A ), share a basic structure with the acetylcholine receptor ionophore that consists of five subunits (see Fig. 4-14C ), with each subunit containing four transmembrane segments (see Fig. 4-14D ).[50] [128] [135] Interactions of anesthetics with particular subunits of these ligand-gated ion channels can be studied by molecular cloning and expression in cells that do not endogenously contain these receptors.[50] [128] [135] The dependence of GABAA receptor subunit composition on the ability of inhaled anesthetics to alter ligand binding[138] or potentiate (see Fig. 4-14B ) GABA-activated chloride
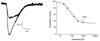 Figure 4-13
The figure on the left shows the current from an oocyte
that expresses the predominant nicotinic acetylcholine receptor subtype (α4β2)
found in the central nervous system. Isoflurane (320 µM) reduces the peak
current obtained in response to 1 µM acetylcholine from 2.4 µA to 1.1
µA (46% of control). The graph on the right shows the dose-response curve
of the inhibition of α4β2 receptor subtype current by isoflurane. Notice
that some inhibition of receptor function occurs even at an isoflurane concentration
of about 0.1 minimum alveolar concentration (MAC). (Adapted from Flood P,
Ramirez-Latorre J, Role L: α4β2 Neuronal nicotinic acetylcholine receptors
in the central nervous system are inhibited by isoflurane and propofol, but α7-type
nicotinic acetylcholine receptors are unaffected. Anesthesiology 86:859, 1997.)
Figure 4-13
The figure on the left shows the current from an oocyte
that expresses the predominant nicotinic acetylcholine receptor subtype (α4β2)
found in the central nervous system. Isoflurane (320 µM) reduces the peak
current obtained in response to 1 µM acetylcholine from 2.4 µA to 1.1
µA (46% of control). The graph on the right shows the dose-response curve
of the inhibition of α4β2 receptor subtype current by isoflurane. Notice
that some inhibition of receptor function occurs even at an isoflurane concentration
of about 0.1 minimum alveolar concentration (MAC). (Adapted from Flood P,
Ramirez-Latorre J, Role L: α4β2 Neuronal nicotinic acetylcholine receptors
in the central nervous system are inhibited by isoflurane and propofol, but α7-type
nicotinic acetylcholine receptors are unaffected. Anesthesiology 86:859, 1997.)
Inhaled anesthetics have an agent-specific effect on 5-HT3 serotonin receptor function and may potentiate or inhibit 5-HT currents.[142] Inhaled anesthetics produce subunit-selective actions on excitatory glutamate receptors, with GluR3 (AMPA subtype) receptors being inhibited or unaffected by anesthetics, whereas GluR6 (kainate subtype) receptor function is enhanced by volatile agents. [143] Mutations of a specific amino acid located in a transmembrane segment of the GluR6 receptor removes the ability of halothane, isoflurane, and enflurane to enhance GluR6 receptor function.[143]
Gaseous anesthetics may have selective actions on excitatory versus inhibitory receptors compared with volatile anesthetics. Many studies indicate that nitrous oxide and xenon inhibit excitatory N-methyl-D-aspartate (NMDA) glutamate transmission while having little or no effect on GABAergic inhibition, [48] [144] [145] with volatile anesthetics tending to exert their greatest effects on GABAergic transmission. Moreover, the gaseous anesthetics cyclopropane and butane (at concentrations sufficient to induce anesthesia) fail to potentiate GABAergic transmission.[134] These findings imply different mechanisms of action for gaseous and volatile anesthetics.
 Figure 4-14
A, The neurotransmitters
γ-aminobutyric acid (GABA) and glycine are released at inhibitory synapses,
bind to their respective receptors, and result in a flow of chloride ions across
the postsynaptic membrane. B, Inhaled anesthetics
potentiate the postsynaptic inhibition produced by GABA or glycine. C,
Inhibitory receptor-channel complexes, as well as many other ligand-gated channels,
typically consist of five subunits embedded in a membrane lipid bilayer. Many subunit
combinations may exist for a given receptor-channel complex. D,
Each of the subunits contains four transmembrane segments, and the second transmembrane
segment of each subunit is thought to line a pore (i.e., channel) that extends across
the membrane. Anesthetic sensitivity can be altered by mutation of critical amino
acids in these transmembrane regions. (Adapted from Franks NP, Lieb WR:
Anaesthetics set their sites on ion channels. Nature 389:334, 1997.)
Figure 4-14
A, The neurotransmitters
γ-aminobutyric acid (GABA) and glycine are released at inhibitory synapses,
bind to their respective receptors, and result in a flow of chloride ions across
the postsynaptic membrane. B, Inhaled anesthetics
potentiate the postsynaptic inhibition produced by GABA or glycine. C,
Inhibitory receptor-channel complexes, as well as many other ligand-gated channels,
typically consist of five subunits embedded in a membrane lipid bilayer. Many subunit
combinations may exist for a given receptor-channel complex. D,
Each of the subunits contains four transmembrane segments, and the second transmembrane
segment of each subunit is thought to line a pore (i.e., channel) that extends across
the membrane. Anesthetic sensitivity can be altered by mutation of critical amino
acids in these transmembrane regions. (Adapted from Franks NP, Lieb WR:
Anaesthetics set their sites on ion channels. Nature 389:334, 1997.)
The nonimmobilizers 1,2-dichlorohexafluorocyclobutane and 2,3-dichlorooctafluorobutane (see Fig. 4-10 ) have little or no effect on GABA, glycine, 5-HT3 , and glutamate receptor function. [50] [143] However, these nonimmobilizing agents are highly effective in blocking currents through neuronal nicotinic acetylcholine receptors,[146] consistent with the concept that anesthetic action on nicotinic acetylcholine receptors may be important for anesthetic-induced behavioral changes (e.g., amnesia) but not important for preventing response to surgical stimulation.[36] [105] [136] [146]
The flux of ions through voltage-gated ion channels is controlled by the electric field across the membrane. These channels share structural similarities and include sodium and potassium channels involved in the propagation of neuronal action potentials and calcium channels that control neuronal excitability and entry of calcium through presynaptic membranes and eventual neurotransmitter release. Although voltage-gated ion channels are often found to be insensitive to even high anesthetic concentrations,[2] [147] it is recognized that pharmacologic effects on a particular channel may vary from tissue to tissue or among channel subtypes. Nitrous oxide inhibits selective (T-type) voltage-gated calcium currents in small sensory neurons at subanesthetic concentrations [148] ; volatile anesthetics (but not the nonimmobilizer 1,2-dichlorohexafluorocyclobutane [see Fig. 4-10 ]) inhibit dorsal root ganglion sodium channels[110] ; and clinical concentrations of volatile anesthetics partially inhibit voltage-dependent potassium currents in human potassium channels expressed in a cell line.[111]
The inhibition of electrical activity by volatile anesthetics in molluscan neurons (see Fig. 4-6 ) is associated with activation of background (not voltage-gated) potassium channels and membrane hyperpolarization.[34] [35] Anesthetic-sensitive background potassium channels are also found in mammals and contain four transmembrane segments with two pore-forming domains.[115] The ability of an inhaled anesthetic to open background potassium channels depends on the subtype of channel examined and is agent specific.[115] Currents through a human potassium channel that is prominent in spinal cord are potentiated by halothane, isoflurane, desflurane, and enflurane, whereas the nonimmobilizer 1,2-dichlorohexafluorocyclobutane (see Fig. 4-10 ) caused slight inhibition.[149]
In contrast to the ionotropic multiple-subunit ligand-gated ion channels where membrane ion permeabilities change within a few milliseconds of agonist binding, metabotropic receptors consist of a single subunit, are coupled to G proteins, and evoke changes in neuronal excitability over hundreds of milliseconds. The ability of halothane but not isoflurane to inhibit muscarinic signaling activated by acetylcholine indicates a variable effect of anesthetics and suggests that muscarinic inhibition may be more relevant to side effects of anesthetics than to anesthetic action per se.[150] The 5-HT type 2A receptor and the metabotropic glutamate receptor mGluR5 are coupled to G proteins and are inhibited by inhaled anesthetics and the nonimmobilizer 1,2-dichlorohexafluorocyclobutane (see Fig. 4-10 ), implying
G proteins are potential membrane sites where anesthetics exert their functional effects. They are guanine nucleotide-binding neuronal membrane proteins that couple many neurotransmitter receptors to ion channels in the brain. The binding of a neurotransmitter to its receptor can influence the activation state of a G protein, which can control the opening or closing of an ion channel. Although evidence for an anesthetic target site on G proteins is supported by the abilities of volatile anesthetics to reduce the exchange of guanine nucleotides and to promote the interaction of G protein α and βγ subunits,[153] the use of halothane photoaffinity labeling failed to demonstrate direct anesthetic binding sites on G protein subunits.[154] Alternatively, anesthetic inhibition of metabotropic receptor function may result from a direct action on the coupled ion channel.[147]
Protein kinase C is an enzyme involved in the phosphorylation of proteins and may modulate neurotransmission through effects on transmitter release and conductance of ions through membrane channels.[153] Protein kinase C exists in several isoforms, and anesthetic effects on this enzyme are complicated, with enzyme activity depending on the isoform and tissue examined, the presence of endogenous enzyme activators, and the particular anesthetic and its duration of treatment.[155] The inhibitory actions of inhaled anesthetics on 5-HT type 2A receptors,[151] metabotropic glutamate mGluR5 receptors,[152] substance P receptors,[156] and selected sodium channels[157] are thought to depend on activation of protein kinase C.
|
|
|
|
|
|
|
|
|
|
|
|
|