PHYSICOCHEMICAL NATURE OF THE SITE OF ANESTHETIC ACTION
The preceding sections suggest that anesthetics may act at several
gross (e.g., spinal cord versus reticular activating system) or microscopic (e.g.,
presynaptic versus postsynaptic) sites. The varied nature of these sites, however,
does not preclude a unique action at a molecular level. For instance, depression
of presynaptic neurotransmitter release and blockade of current flow through the
postsynaptic membrane may arise from an anesthetic perturbation at an identical molecular
site, even though the geographic locations of these sites differ. The concept that
all inhaled anesthetics have a common mode of action on a specific molecular structure
is called the unitary theory of narcosis. The nature
of this presumed common site has been explored by correlating the physical properties
of anesthetics with their potencies. The rationale behind this approach is that
the best correlation between anesthetic potency and a physical property suggests
the nature of the anesthetic site of action. For example, the correlation of MAC
and lipid solubility implies that the site of action is hydrophobic. The correlations
that depend on forces exerted between anesthetic molecules (e.g., the boiling point
of an anesthetic) are not important to the study of anesthetic mechanisms; as such,
intermolecular forces cannot be representative of a single site of action. These
correlations are defined by the interaction of each anesthetic with itself rather
than with a common site.
Hydrophobic Site: The Meyer-Overton
Rule
The physical property that correlates best with anesthetic potency
is lipid solubility ( Table 4-2
and Fig. 4-9
).[87]
[88]
[89]
This
correlation
is called the Meyer-Overton rule, after its two discoverers.
For most inhaled agents, the product of the anesthetizing partial pressure and its
olive oil-gas partition coefficient varies little over approximately a 100,000-fold
range of anesthetizing partial pressures (see Table
4-2
and Fig. 4-9
).
For the correlation to be perfect, this product would have to be the same for all
anesthetics for a given animal. Within a given species, an even better correlation
between anesthetic potency and solubility may be obtained with solvents that are
better characterized than olive oil, such as lecithin, an amphipathic phospholipid
with hydrophobic and polar components.[90]
The
closeness of this correlation implies a unitary molecular site of action and suggests
that anesthesia results when a specific number of anesthetic molecules occupy a crucial
hydrophobic region in the CNS. This finding has led many investigators to look for
the molecular basis of anesthetic action in cellular hydrophobic regions.
TABLE 4-2 -- Oil-gas partition coefficients and potencies of inhaled anesthetics in dogs,
humans, and mice
|
|
Dogs |
Humans |
Mice |
|
Anesthetic |
Oil-Gas Partition Coefficient
(37°C) |
MAC (atm) |
MAC × Oil-Gas (atm) |
MAC (atm) |
MAC × Oil-Gas (atm) |
Righting Reflex ED50
(atm) |
Righting Reflex ED50
×
Oil-Gas (atm) |
|
Thiomethoxyflurane |
7230 |
0.00035 |
2.53 |
|
|
|
|
|
Methoxyflurane |
970 |
0.0023 |
2.23 |
0.0016 |
1.55 |
0.0023 |
2.23 |
|
Dioxychlorane |
1286 |
0.0011 |
1.41 |
|
|
0.0033 |
4.24 |
|
Chloroform |
265 |
0.0077 |
2.08 |
|
|
0.00357 |
0.95 |
|
Halothane |
224 |
0.0087 |
1.95 |
0.0074 |
1.66 |
0.00645 |
1.45 |
|
Enflurane |
96.5 |
0.0267 |
2.58 |
0.0168 |
1.62 |
0.0123 |
1.19 |
|
Isoflurane |
90.8 |
0.0141 |
1.28 |
0.0115 |
1.04 |
0.00663 |
0.60 |
|
Compound 485 |
25.8 |
0.125 |
3.23 |
|
|
|
|
|
Desflurane |
18.7 |
0.072 |
1.35 |
0.060 |
1.12 |
0.045 |
0.84 |
|
HFCICOCHFCF3
|
96.6 |
0.0224 |
2.16 |
|
|
|
|
|
Iso-Indoklon |
27.0 |
0.460 |
1.24 |
|
|
0.0265 |
0.72 |
|
Aliflurane |
124 |
0.0184 |
2.28 |
|
|
|
|
|
Synthane |
95 |
0.012 |
1.14 |
|
|
|
|
|
Diethyl ether |
65 |
0.0304 |
1.98 |
0.0192 |
1.25 |
0.032 |
2.08 |
|
Fluroxene |
47.7 |
0.0599 |
2.86 |
0.034 |
1.62 |
0.0345 |
1.65 |
|
Sevoflurane |
47.2 |
0.0236 |
1.11 |
0.0205 |
0.97 |
0.0116 |
0.58 |
|
Cyclopropane |
11.8 |
0.175 |
2.06 |
0.092 |
1.09 |
0.142 |
1.68 |
|
Xenon |
1.9 |
1.19 |
2.26 |
0.71 |
1.35 |
0.95 |
1.80 |
|
Ethylene |
1.26 |
|
|
0.67 |
0.84 |
1.30 |
1.64 |
|
Nitrous oxide |
1.4 |
1.88 |
2.63 |
1.04 |
1.46 |
1.54 |
2.16 |
|
Krypton |
0.5 |
|
|
|
|
4.5 |
2.25 |
|
Sulfur hexafluoride |
0.293 |
4.9 |
1.44 |
|
|
5.4 |
1.58 |
|
Argon |
0.15 |
|
|
|
|
15.2 |
2.28 |
|
Carbon tetrafluoride |
0.073 |
26 |
1.90 |
|
|
18.7 |
1.36 |
|
Nitrogen |
0.072 |
≥43.5 |
≥3.13 |
|
|
34.3 |
2.47 |
|
Mean ± SE |
|
2.04 ± 0.14 |
1.30 ± 0.08 |
1.69 ± 0.19 |
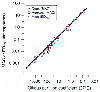 Figure 4-9
Correlation of the minimum alveolar concentration (MAC)
in humans and dogs and the righting-reflex 50% effective dose (ED50
) in
mice with lipid solubility (i.e., the olive oil-gas partition coefficient). Values
are taken from Table 4-2
.
Figure 4-9
Correlation of the minimum alveolar concentration (MAC)
in humans and dogs and the righting-reflex 50% effective dose (ED50
) in
mice with lipid solubility (i.e., the olive oil-gas partition coefficient). Values
are taken from Table 4-2
.
 Figure 4-9
Correlation of the minimum alveolar concentration (MAC)
in humans and dogs and the righting-reflex 50% effective dose (ED50
) in
mice with lipid solubility (i.e., the olive oil-gas partition coefficient). Values
are taken from Table 4-2
.
Figure 4-9
Correlation of the minimum alveolar concentration (MAC)
in humans and dogs and the righting-reflex 50% effective dose (ED50
) in
mice with lipid solubility (i.e., the olive oil-gas partition coefficient). Values
are taken from Table 4-2
.