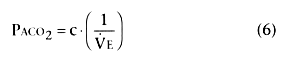GAS EXCHANGE
The major function of the lung is gas exchange: the addition
of O2
to blood and the elimination of CO2
from blood. Because
of the close relationship between the partial pressure of CO2
in blood
(PCO2
) and arterial (and hence tissue)
pH, the goal is maintenance of arterial CO2
within an appropriate physiologic
range. Adequate O2
delivery must also be maintained. Figure
36-1
shows the O2
cascade from atmospheric gas to the intracellular
site of use. Assessment of the adequacy of this delivery system may be made at any
step of the cascade. For example, common clinical practice dictates that arterial
O2
content (PaO2
) should be
sufficient. It is common practice to attempt to maintain arterial hemoglobin (Hb)
O2
saturation (SaO2
) level
above 90%, a reasonable and practical goal for two reasons. First, clinical experience
supports the notion that maintenance of hemoglobin at 90% saturation with O2
in the presence of adequate cardiac output can provide sufficient O2
delivery
to the tissues. Second, because the Hb-O2
dissociation curve becomes
abruptly steeper at O2
saturation levels below 90% (typical PO2
of 55 to 60 mm Hg), further decreases in PaO2
may result in sharp diminution of arterial O2
content.
In addition to SaO2
,
O2
delivery is a function of blood flow and hemoglobin concentration.
A target value for SaO2
of 90% or greater
should therefore not become a sacrosanct standard for all conditions. Acute altitude
hypoxia with SaO2
values between 50% and
70% is well tolerated by normal individuals,[5]
[6]
even during exercise, for periods of several
hours or days, with no loss of consciousness, preservation of cardiac function, and
minimal or no evidence of permanent sequelae.[7]
[8]
[9]
[10]
[11]
Individuals with acute anemia may tolerate
hypoxia less well because of the enhanced reduction in arterial O2
content.
Alveolar Gases
The lung consists of a heterogeneous collection of gas exchange
units (alveoli), with a range of O2
and CO2
gas tensions.
It is therefore erroneous to speak of "the" alveolar PO2
or PCO2
. However, the concept of a homogeneous
lung, in which all alveoli have the same gas tensions, is a useful one. The alveolar
partial pressures calculated by using such a model may be thought of as averages
for the O2
and CO2
partial pressures in the real (nonhomogeneous)
lung. Equations for alveolar PO2
and
PCO2
(PAO2
and PACO2
), or alveolar gas equations,
are as follows:
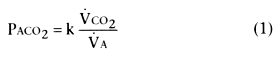
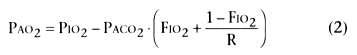
In these equations, V̇CO2
is the CO2
production rate, V̇O2
is the O2
consumption rate, V̇A is the alveolar ventilation
rate, k is a constant, FIO2
is the fractional
inspired O2
concentration, PIO2
is the inspired PO2
after humidification
or (Pbarometric
− PH2O
) · FIO2
,
and R is the respiratory exchange ratio or V̇CO2
/V̇O2
(typically about 0.8).
An approximation to the O2
alveolar gas equation follows:
PAO2
 PIO2
− 1.2 × PCO2
(3)
PIO2
− 1.2 × PCO2
(3)
Unlike the alveolar equation for O2
, the equation for
PACO2
is a function of only two variables.
Because in clinical medicine V̇CO2
is relatively constant, PACO2
is therefore
mainly a function of alveolar ventilation V̇A,
to which it is inversely proportional.
V̇A = V̇E
− V̇D (4)
In Equation 4, V̇E is the respiratory minute
ventilation, and V̇D is dead space ventilation.
Because alveolar ventilation is usually a constant fraction of minute ventilation,
the following relationship applies:
V̇A = k' ·
V̇E (5)
The alveolar gas equation for CO2
may be rewritten
as follows:
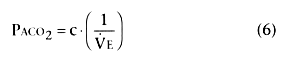
In this form of the equation, c is a constant derived from constants k and k'.
This approximation may not hold if CO2
production is
substantially elevated, such as during a major motor seizure, shivering, or fever.
CO2
production may decrease as a result of general anesthesia or hypothermia.
It is altered by approximately 7% for each 1°C change in body temperature.
In young individuals, it has been observed to increase by 100% to 300% during violent
shivering,[12]
although in elderly patients (>60
years), the observed increase is only about 38%.[13]
The alveolar gas equation for O2
(Equation 2) can be
used to delineate the factors that result in low PAO2
.
Several major factors apply:
- Low PIO2
caused by decreased
barometric pressure (altitude) or breathing a gas mixture with inspired O2
fraction (FIO2
) less than 0.21
- Elevated PACO2
caused by hypoventilation
or increased CO2
production
- Reduced respiratory exchange ratio (R), which is usually a minor effect
because the physiologic range of R is on the order of 0.7 to 1.2, with the latter
value seen during acute metabolic acidosis or excessive feeding.
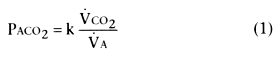
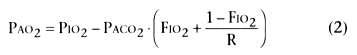
![]() PIO2
− 1.2 × PCO2
(3)
PIO2
− 1.2 × PCO2
(3)