|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Although no special monitoring or anesthetic technique is required for the patient with a pacemaker, attention must be given to a number of concerns. First, electrocardiographic monitoring of the patient must include the ability to detect pacemaker discharges. Currently, most electrocardiographic monitors in both the operating room and the intensive care unit perform digital acquisition and analysis of electrocardiographic signals. As a result, in their default settings, these monitors will filter the pacemaker artifacts, and no pacemaker "spikes" will
| ELA Medical |
| Brio (212, 220, 222) |
| Chorus RM (7034, 7134) |
| Opus RM (4534) |
| Talent (130, 213, 223) |
| Guidant Medical and CPI Cardiac Pacemakers, Inc. |
| Pulsar (1172, 1272) |
| Pulsar Max (1170, 1171, 1270) |
| Pulsar Max II (1180, 1181, 1280) |
| Insignia Plus (1194, 1297, 1298) |
| Medtronic |
| Kappa 400 series (KDR401, KDR403, KSR401, KSR403) |
| Telectronics/St. Jude |
| Meta (1202, 1204, 1206, 1230, 1250, 1254, 1256) |
| Tempo (1102, 1902, 2102, 2902) |
Second, patient monitoring must include the ability to ensure that paced electrical activity is converted to mechanical systoles. Mechanical systoles are best evaluated by pulse oximetry plethysmography or arterial pressure waveform display.
Third, there is limited experience with intraoperative BiV pacing at this time. These patients often have ejection fractions less than 30%, and they depend on pacing in both ventricles to improve their cardiac output. Loss of ventricular pacing from any cause (AV dyssynchrony [atrial fibrillation, atrial flutter, appearance of junctional rhythm], myocardial ischemia, acid-base disturbance, change in pacing threshold, ESU interference, and so on) can cause an immediate decrease in cardiac output. Monitoring of these patients probably should include beat-to-beat monitoring of cardiac output. The patient with HOCM pacing is also dependent upon forced ventricular pacing to limit left ventricular outflow tract obstruction.
Fourth, some patients might need an increased pacing rate during the perioperative period to meet an increased oxygen demand. This subject is often not addressed. Pacemaker patients reportedly have high postoperative morbidity and mortality,[51] and failure to address tissue oxygen demands and cardiac output needs might contribute to this problem.
Fifth, appropriate equipment must be on hand to provide backup pacing and/or defibrillation to the patient who might need it. The literature and anecdotal experience suggest that cardiac generators, while hardy, occasionally perform some untoward maneuver or fail, even in the absence of EMI.[52] Acceptable, but inappropriate behavior of a pacemaker or ICD can create an inhospitable situation. Even a properly working, dual chamber pacemaker can produce R-on-T pacing, especially in the setting of a junctional rhythm or PVCs ( Fig. 35-6 ).
The medical team caring for the patient with an implanted cardiac pulse generator must understand that the patient has been deemed needy of this device by a physician who is an expert in the diagnosis and management of cardiac rhythm issues. Few anesthesiologists are qualified to contradict this diagnosis, yet some persist in providing an anesthetic without appropriate backup pacing and defibrillation equipment on hand.
Monopolar "Bovie" electrosurgery (ESU) use remains the principal intraoperative issue for the patient with a pacemaker. Between 1984 and 1997, the U.S. FDA was notified of 456 adverse events with pulse generators, 255 from electrosurgery, and a "significant number" of device failures.[53] Monopolar ESU is more likely to cause problems than bipolar ESU, and patients with unipolar electrode configuration are more sensitive to electromagnetic interference than those with bipolar configurations.[54] The most common effect of ESU on pacemakers is ventricular oversensing which causes pacemaker inhibition (see Fig. 35-5B ). Sometimes, the pacemaker determines that significant EMI is present and begins pacing asynchronously at the programmed lower rate.[55] This behavior is called "noise reversion mode pacing," even though the pacemaker does not actually change "modes."
Magnet placement during electrosurgery might prevent aberrant pacemaker behavior, and it might allow reprogramming of an older (pre-1990) generator. Note that not all generators "open" their programming window during placement of a magnet (for example, devices from Guidant and/or CPI cannot be programmed in the presence of a magnet). Newer generators are believed to be relatively immune to spurious reprogramming from EMI.
If monopolar electrosurgery is to be used, then the electrosurgical current-return pad (often misidentified as the "grounding pad") must be placed to ensure that the electrosurgical current path does not cross the pacemaking system. Some authors recommend placement of this pad on the shoulder for head and neck procedures or the distal arm (with sterile draping of the wire) for breast and
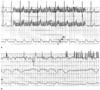 Figure 35-5
Disabling the pacemaker artifact filter on a digitally
processed electrocardiographic (ECG) monitor results in the "painting" of environmental
interference (EMI) as pacemaker artifacts. A, The
patient's underlying rate exceeded the pacemaker's programmed lower rate limit, and
no pacing took place. However, activation of the monopolar electrosurgical unit
(ESU) in the "cut" mode produced sufficient electromagnetic noise that the monitor
began painting pacemaker artifacts at a rate of about 20 Hz. The top
tracing is ECG lead II, the middle tracing
is ECG lead V5, and the bottom tracing is the invasive
arterial pressure waveform. B, "Coagulation" ESU
produced ventricular oversensing with pacemaker inhibition and left this patient
with a compromised cardiac output. There is also evidence of inappropriate monitor
painting of pacemaker artifacts from the EMI. The top tracing
is ECG lead II, the middle tracing is the pulse oximeter
plethysmogram, and the bottom tracing is the invasive
arterial pressure waveform. (Adapted from Rozner MA: [untitled letter].
Pacing Clin Electrophysiol 26:923–925, 2003.)
Figure 35-5
Disabling the pacemaker artifact filter on a digitally
processed electrocardiographic (ECG) monitor results in the "painting" of environmental
interference (EMI) as pacemaker artifacts. A, The
patient's underlying rate exceeded the pacemaker's programmed lower rate limit, and
no pacing took place. However, activation of the monopolar electrosurgical unit
(ESU) in the "cut" mode produced sufficient electromagnetic noise that the monitor
began painting pacemaker artifacts at a rate of about 20 Hz. The top
tracing is ECG lead II, the middle tracing
is ECG lead V5, and the bottom tracing is the invasive
arterial pressure waveform. B, "Coagulation" ESU
produced ventricular oversensing with pacemaker inhibition and left this patient
with a compromised cardiac output. There is also evidence of inappropriate monitor
painting of pacemaker artifacts from the EMI. The top tracing
is ECG lead II, the middle tracing is the pulse oximeter
plethysmogram, and the bottom tracing is the invasive
arterial pressure waveform. (Adapted from Rozner MA: [untitled letter].
Pacing Clin Electrophysiol 26:923–925, 2003.)
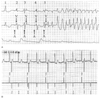 Figure 35-6
Normal dual chamber pacemaker timing can produce R-on-T
pacing. A, This strip demonstrates functional ventricular
undersensing of a premature ventricular contraction (PVC) with a resultant R-on-T
pace leading to torsades de pointes. This patient had a dual chamber pacemaker in
the DDD mode with a programmed lower rate of 70 beats/min (R-R interval is 857 msec)
and an atrioventricular delay of 200 msec. With these parameters, the pacemaker
paces the atrium at 657 msec after any previous ventricular event. Atrial pacing
(A) and ventricular pacing (V) are indicated. The top tracing
is electrocardiographic (ECG) lead II, the middle tracing
is ECG lead V5, and the bottom tracing is the invasive
arterial blood pressure. Approximately 660 msec after the first QRS (1) on the strip
(which was adequately sensed by the pacemaker), an atrial stimulus is emitted. At
200 msec after this atrial pace, a ventricular stimulus is emitted, appearing to
depolarize the ventricle (2). About 660 msec later (3), the patient had a PVC.
Because the pacemaker was preparing to emit the atrial stimulus, it had disabled
its ventricular sensing element and failed to sense this PVC (i.e., functional undersensing).
At 200 msec after the atrial stimulus, no ventricular event had been sensed, and
the pacemaker emitted a ventricular stimulus on the T wave. Because the ventricle
was in a refractory period from the PVC, there was no depolarization of the ventricle
(i.e., functional noncapture). At 660 msec from this attempted ventricular pacing,
the pacemaker again paces the atrium (4), and it appears that the next ventricular
pacing impulse captures the ventricle. At (5), there is a repeat of the events at
(3); the pacemaker disabled its sensing elements in preparation to pace the atrium
and failed to detect the PVC. This time, however, the ventricular pace on the T
wave produced torsades de pointes. B, This strip
was obtained from a Medtronic programmer during interrogation of a Kappa 700 dual-chamber
pacemaker. The top tracing is ECG lead II, and the
bottom tracing is the marker channel, which shows
the pacemaker's interpretation of events. This pacemaker was programmed to the DDD
mode with a lower rate of 60 beats/min. The atrioventricular (AV) delay was 200
msec. As a result, after any ventricular event, the pacemaker will emit an atrial
pulse at 800 msec if no intervening atrial or ventricular event takes place. This
patient had a junctional rhythm at 75 beats/min (corresponding to an R-R interval
of 800 msec), and the pacemaker emitted an atrial pulse just as the junctional event
occurred. Because the pacemaker disables its ventricular sensing element when emitting
the atrial pulse, it failed to detect the ventricular event and emitted the ventricular
pulse 200 msec later, falling on the T wave. This inappropriate pacing takes place
every other cycle, because every other junctional event is sensed about 600 msec
after the previous ventricular pace. Decreasing the AV delay decreases the likelihood
of pacing during the vulnerable period of the ventricle. Atrial pace (AP), ventricular
pace (VP), and ventricular sensed event (VS) are indicated. The third complex deserves
comment. The pacemaker sensed this ventricular event as it re-enabled its sensing
element, and the pacemaker could not tell whether the sensed event was a true ventricular
depolarization or an echo of the atrial pace (called far-field oversensing). When
a signal from the ventricle is sensed within 30 to 90 msec after an atrial pace,
many pacemakers immediately emit a ventricular pacing stimulus. Called a ventricular
safety pace, this pacing stimulus is designed to protect the patient from
inappropriate sensing of the atrial signal by the ventricular channel, which would
then inhibit the ventricular output. The safety pace is emitted at 110 msec to prevent
R-on-T pacing. This feature is also called nonphysiologic AV
delay by some manufacturers. R-on-T pacing can be appropriate (but not
ideal) behavior of a DDD or DDI pacemaker in the setting of PVCs or a junctional
rhythm. It can also be seen with atrial or ventricular undersensing.
Figure 35-6
Normal dual chamber pacemaker timing can produce R-on-T
pacing. A, This strip demonstrates functional ventricular
undersensing of a premature ventricular contraction (PVC) with a resultant R-on-T
pace leading to torsades de pointes. This patient had a dual chamber pacemaker in
the DDD mode with a programmed lower rate of 70 beats/min (R-R interval is 857 msec)
and an atrioventricular delay of 200 msec. With these parameters, the pacemaker
paces the atrium at 657 msec after any previous ventricular event. Atrial pacing
(A) and ventricular pacing (V) are indicated. The top tracing
is electrocardiographic (ECG) lead II, the middle tracing
is ECG lead V5, and the bottom tracing is the invasive
arterial blood pressure. Approximately 660 msec after the first QRS (1) on the strip
(which was adequately sensed by the pacemaker), an atrial stimulus is emitted. At
200 msec after this atrial pace, a ventricular stimulus is emitted, appearing to
depolarize the ventricle (2). About 660 msec later (3), the patient had a PVC.
Because the pacemaker was preparing to emit the atrial stimulus, it had disabled
its ventricular sensing element and failed to sense this PVC (i.e., functional undersensing).
At 200 msec after the atrial stimulus, no ventricular event had been sensed, and
the pacemaker emitted a ventricular stimulus on the T wave. Because the ventricle
was in a refractory period from the PVC, there was no depolarization of the ventricle
(i.e., functional noncapture). At 660 msec from this attempted ventricular pacing,
the pacemaker again paces the atrium (4), and it appears that the next ventricular
pacing impulse captures the ventricle. At (5), there is a repeat of the events at
(3); the pacemaker disabled its sensing elements in preparation to pace the atrium
and failed to detect the PVC. This time, however, the ventricular pace on the T
wave produced torsades de pointes. B, This strip
was obtained from a Medtronic programmer during interrogation of a Kappa 700 dual-chamber
pacemaker. The top tracing is ECG lead II, and the
bottom tracing is the marker channel, which shows
the pacemaker's interpretation of events. This pacemaker was programmed to the DDD
mode with a lower rate of 60 beats/min. The atrioventricular (AV) delay was 200
msec. As a result, after any ventricular event, the pacemaker will emit an atrial
pulse at 800 msec if no intervening atrial or ventricular event takes place. This
patient had a junctional rhythm at 75 beats/min (corresponding to an R-R interval
of 800 msec), and the pacemaker emitted an atrial pulse just as the junctional event
occurred. Because the pacemaker disables its ventricular sensing element when emitting
the atrial pulse, it failed to detect the ventricular event and emitted the ventricular
pulse 200 msec later, falling on the T wave. This inappropriate pacing takes place
every other cycle, because every other junctional event is sensed about 600 msec
after the previous ventricular pace. Decreasing the AV delay decreases the likelihood
of pacing during the vulnerable period of the ventricle. Atrial pace (AP), ventricular
pace (VP), and ventricular sensed event (VS) are indicated. The third complex deserves
comment. The pacemaker sensed this ventricular event as it re-enabled its sensing
element, and the pacemaker could not tell whether the sensed event was a true ventricular
depolarization or an echo of the atrial pace (called far-field oversensing). When
a signal from the ventricle is sensed within 30 to 90 msec after an atrial pace,
many pacemakers immediately emit a ventricular pacing stimulus. Called a ventricular
safety pace, this pacing stimulus is designed to protect the patient from
inappropriate sensing of the atrial signal by the ventricular channel, which would
then inhibit the ventricular output. The safety pace is emitted at 110 msec to prevent
R-on-T pacing. This feature is also called nonphysiologic AV
delay by some manufacturers. R-on-T pacing can be appropriate (but not
ideal) behavior of a DDD or DDI pacemaker in the setting of PVCs or a junctional
rhythm. It can also be seen with atrial or ventricular undersensing.
|
|
|
|
|
|
|
|
|
|
|
|
|