Treatment of Arrhythmias
The diagnosis and treatment of arrhythmias can be simplified by
using the following six questions as a checklist when looking at an electrocardiographic
display and attempting to decide whether treatment is necessary:
- What is the heart rate?
- Is the rhythm regular?
- Is there one P wave for each QRS complex?
- Is the QRS complex normal?
- Is the rhythm dangerous?
- Does the rhythm require treatment?
Following are some common intraoperative arrhythmias to which
the six questions should be applied.
Sinus Bradycardia
The pacemaker site is in the sinus node, but the rate is slower
than normal. Etiologic factors include drug effects, acute inferior myocardial infarction,
hypoxia, vagal stimulation, and high sympathetic blockade. Sinus bradycardia accounts
for 11% of intraoperative arrhythmias:
- Heart rate: The heart rate is slower than
60 beats/min. In patients on chronic β-blocker therapy, it is defined as a
heart rate of less than 50 beats/min.
- Rhythm: The rhythm is regular, except
for occasional escape beats from other pacemaker sites.
- P:QRS: There is a 1:1 relationship between
the P waves and the QRS complexes.
- QRS complex: Normal.
- Significance: Heart rates lower than 40
beats/min are poorly tolerated even in healthy patients and should be evaluated on
the basis of their effect on cardiac output. Treatment is recommended if hypotension,
ventricular arrhythmias, or signs of poor peripheral perfusion are observed. Sinus
bradycardia may be part of the sick sinus syndrome in which sinus node dysfunction
can precipitate bradycardias, heart block, tachyarrhythmias, or alternating bradyarrhythmias
and tachyarrhythmias ( Fig. 34-13
).
[38]
- Treatment: Usually, none is necessary.
A progression from atropine (0.5 to 1.0 mg by intravenous bolus, repeated every
3 to 5 minutes, up to 0.04 mg/kg or approximately a 3.0-mg total dose for the average
75-kg male patient), to ephedrine (5 to 25 mg by intravenous bolus), dopamine (5
to 20 µg/kg/min by intravenous infusion), epinephrine (2 to 10 µg/min
by intravenous infusion), or isoproterenol (2 to 10 µg/min by intravenous infusion)
to temporary transcutaneous pacing or transvenous pacemaker insertion may be necessary
for severe or refractory sinus bradycardia. Immediate institution of transcutaneous
pacing is especially important if the patient is symptomatic.
Sinus Tachycardia
The pacemaker site is in the sinus node, but the rate is faster
than normal. Sinus tachycardia is the most commonly occurring arrhythmia in the
perioperative period. It occurs with such frequency that it is not included in most
incidence studies. Common causes include pain, inadequate anesthesia, hypovolemia,
fever, hypoxia, hypercarbia, heart failure, and drug effects.[4]
- Heart rate: The rate is faster than 100
beats/min in the adult patient and can go up to 170 beats/min, which may be seen
during a severe episode of hyperpyrexia.
- Rhythm: Regular.
- P:QRS: 1:1.
- QRS complex: Normal. There may be associated
ST-segment depression with severe increases in heart rate and resulting myocardial
ischemia.
- Significance: Prolonged tachycardias in
patients with underlying heart disease can precipitate congestive heart failure because
of the increased myocardial
work required. The tachycardia decreases coronary perfusion time, which can cause
secondary ST-T wave changes and can precipitate angina pectoris in patients with
coronary artery disease. A major diagnostic problem is encountered when the heart
rate is 150 beats/min, because this is a common rate for sinus tachycardia, paroxysmal
atrial tachycardia (PAT), or atrial flutter with a 2:1 block. These three arrhythmias
can sometimes be separated by the use of carotid sinus massage, intravenous administration
of edrophonium, or atrial or esophageal electrocardiographic leads to identify the
P waves on the ECG more accurately.
- Treatment: The underlying disorder should
be treated. Hypovolemia and light anesthesia are most common causes. If necessary,
in patients with ischemic heart disease who develop tachycardia and ST-segment changes,
after excluding hypovolemia and while determining the cause, esmolol should be used
to prevent further myocardial ischemia.
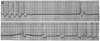 Figure 34-13
Sick sinus syndrome. (From Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.)
Figure 34-13
Sick sinus syndrome. (From Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.)
Sinus Arrhythmia
The pacemaker impulse arises from the sinoatrial node, but the
arrhythmia is manifested by alternating periods of slower and faster heart rates.
The PR interval is normal, as is the QRS complex. Most commonly, but not invariably,
the rate increases with inspiration and decreases with expiration. This arrhythmia
occurs more often in children than in adults.[4]
- Heart rate: 60 to 100 beats/min.
- Rhythm: Irregular.
- P:QRS: 1:1.
- QRS complex: Normal.
- Significance: Normal finding.
- Treatment: None.
Atrial Premature Beats
An ectopic pacemaker site in the left or the right atrium initiates
the atrial premature beat (APB). The shape of the P wave is different from the usual
sinoatrial node P wave and may be inverted. The PR interval may be shorter or longer
than normal, depending on the site of the ectopic focus and on the refractoriness
of the atrioventricular nodal pathway. The APB spreads through the atrioventricular
node and ventricular conduction system and, in retrograde fashion, reaches the sinoatrial
node, resetting the sinus pacemaker. The interval from the APB to the next sinus
beat is therefore a normal sinus cycle (i.e., no compensatory pause). The absence
of a compensatory pause is an important distinguishing feature between APBs and VPBs.
Occasionally, APBs may find part of the ventricular conduction system refractory.
In that case, they travel down an aberrant pathway and create an abnormal QRS complex.
They are then called APBs with aberrant ventricular conduction and can easily be
confused with VPBs. Because the recovery period of the right ventricular conduction
system outlasts that of the left, the most common form of aberration appears as a
right bundle branch block (RBBB). Helpful points in separating APBs with aberrant
ventricular conduction from VPBs include (1) the presence of a preceding P wave,
usually abnormally shaped; (2) an RBBB configuration of the QRS complex; (3) the
presence of an rsR' ventricular complex in V1
; and (4) the finding that
the initial vector forces are identical to the preceding beat but are usually the
opposite with a VPB. Other characteristics of APBs are as follows:
- Heart rate: Variable, depending on the
frequency of APBs.
- Rhythm: Irregular.
- P:QRS: Usually 1:1. The P waves have
various shapes and may even be lost in the QRS or T waves. Occasionally, the P wave
is so early as to find the ventricle refractory, and a nonconducted beat occurs.
- QRS complex: Usually normal unless there
is ventricular aberration.
- Significance: In one study, APBs represented
10% of all intraoperative arrhythmias. They have little clinical significance, but
frequent APBs may lead to other more serious supraventricular arrhythmias or may
be a sign of digitalis intoxication.
- Treatment: Rarely necessary, but digitalis,
β-blockers, or verapamil may be considered if hemodynamic function is impaired.
Paroxysmal Supraventricular Tachycardia
Paroxysmal SVT (PSVT) is characterized by a rapid regular rhythm,
usually with a narrow QRS complex and lacking
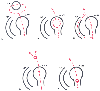 Figure 34-14
Diagrammatic representation of various mechanisms of
supraventricular tachycardia. A-VN, A-V node; BP, bypass tract; S-AN, sinus node.
(Adapted from Marriott HJL: Practical Electrocardiography, 7th ed. Baltimore,
Williams & Wilkins, 1983.)
Figure 34-14
Diagrammatic representation of various mechanisms of
supraventricular tachycardia. A-VN, A-V node; BP, bypass tract; S-AN, sinus node.
(Adapted from Marriott HJL: Practical Electrocardiography, 7th ed. Baltimore,
Williams & Wilkins, 1983.)
the normal sinoatrial node P wave. The inclusion of tachycardias involving the atrioventricular
node ( Fig. 34-14
) allows
their useful classification as caused by reentry in the atrioventricular node, apparent
or concealed accessory atrioventricular pathways, or less often, sinoatrial node
reentry ( Table 34-4
). Ectopic
atrial or ectopic nodal tachycardias are also among the less frequent SVTs. Inappropriate
or persistent sinus tachycardia is another variant. PSVT rhythms are usually abrupt
in onset and termination. PSVT is easily distinguished from rapid atrial fibrillation,
which is an irregular rhythm, or from rapid atrial flutter, which has flutter waves.
- Heart rate: 130 to 270 beats/min.
- Rhythm: Usually regular unless the impulse
originates from multiple atrial foci.
- P:QRS: There is a 1:1 relationship, although
the P wave may often be hidden in the QRS complex or T wave.
- QRS complex: Generally normal, but ST-T
changes indicative of ischemia may be observed. Aberration of ventricular conduction
may occur, complicating the differential diagnosis with ventricular tachycardia.
SVT may also be confused with sinus tachycardia, atrial flutter, and atrial fibrillation.
In differentiating these rhythms, carotid sinus massage or edrophonium (5 to 10
mg given intravenously) traditionally was used. More recently, adenosine (6 to 12
mg by intravenous bolus) has been used to slow the rate by transiently enhancing
the normal degree of atrioventricular block or terminate the arrhythmia.[39]
Esophageal electrocardiographic leads may also be helpful to better define atrial
activity.[40]
- Significance: PSVT can be seen in 5% of
normal young adults and in patients with Wolff-Parkinson-White syndrome or other
preexcitation syndromes. During anesthesia, PSVT accounts for up to 2.5% of all
arrhythmias, and the arrhythmia has been associated with intrinsic heart disease,
systemic illness, thyrotoxicosis, digitalis toxicity, pulmonary embolism, and pregnancy.
When a patient is under anesthesia, PSVT can be precipitated by changes in the autonomic
nervous system tone, by drug effects, or by intravascular
volume shifts and can produce severe hemodynamic deterioration. Sometimes, the PSVT
may be associated with atrioventricular block because of the fast atrial rate and
slow atrioventricular conduction. PSVT with 2:1 block represents digitalis intoxication
in many patients.
- Treatment: This arrhythmia often must
be treated because of its rapid rate and associated poor hemodynamic function. One
or several of the following can be undertaken to treat this arrhythmia:
- Vagal maneuvers such as carotid sinus massage should only be applied to
one side.[41]
[42]
[43]
- Adenosine, which is the drug of choice, is given by 6-mg rapid intravenous
bolus, preferably through an antecubital or more central vein. If no response is
elicited, second and third doses of 12 to 18 mg of adenosine may be administered
by rapid intravenous bolus.[44]
- Verapamil (2.5 to 10 mg given intravenously) terminates atrioventricular
nodal reentry successfully in about 90% of cases. This was the drug of choice and
is now the second choice.[45]
- Amiodarone (150 mg infusion over 10 minutes for the loading dose) is a
recent addition.[46]
- Esmolol (1 mg/kg by bolus and 50 to 200 mg/kg/min by infusion) has been
shown effective.[47]
- Edrophonium (5 to 10 mg by intravenous bolus) can be given.[48]
- Phenylephrine (100 µg by intravenous bolus) is administered if the
patient is hypotensive.[48]
- Intravenous digitalization is performed with one of the short-acting digitalis
preparations: ouabain (0.25 to 0.5 mg given intravenously) or digoxin (0.5 to 1.0
mg given intravenously).[49]
- Rapid overdrive pacing may be done in an effort to capture the ectopic
focus.[50]
- Synchronized cardioversion may be performed with incremental doses of energy
of 100, 200, 300, and 360 J, preferably after light sedative premedication with a
benzodiazepine.[51]
- Procainamide is used in addition to cardioversion or antitachycardia pacing.
Procainamide may be useful to treat SVT when antegrade conduction across an accessory
atrioventricular path is the suspected mechanism.
Electrode catheter ablation using radiofrequency energy has evolved
as the definitive, long-term treatment for most persistent atrioventricular reentrant
or focal atrial SVT.[52]
Atrial Flutter
Atrial flutter most commonly represents a macro-reentrant arrhythmia
that circulates in a specific manner in the right atrium (i.e., counterclockwise
rotation as viewed in the angiographic left anterior oblique view). Because it is
associated with very fast heart rates, it is usually accompanied by atrioventricular
block. Classic sawtooth flutter waves (F waves) are usually present. The characteristics
of atrial flutter are as follows:
- Heart rate: The atrial heart rate is 250
to 350 beats/min with a ventricular rate of about 150 beats/min (2:1 or 3:1 atrioventricular
conduction block).
- Rhythm: The atrial rhythm is regular.
The ventricular rhythm may be regular if a fixed atrioventricular block is present
or irregular if a variable block exists.
- P:QRS: Usually, there is a 2:1 block with
an atrial rate of 300 beats/min and a ventricular rate of 150 beats/min, but it may
vary between 2:1 and 8:1. F waves are best seen in leads V1
, II, and
the esophageal lead.
- QRS complex: Normal. T waves are lost
in the f waves.
- Significance: It usually indicates the
presence of severe heart disease. It is seen with increased incidence in patients
with coronary artery disease, mitral valve disease, pulmonary embolism, hyperthyroidism,
cardiac trauma, cancers of the heart, and myocarditis.
- Treatment: Pharmacologic or synchronized
DC cardioversion, when indicated, should be performed only after careful consideration
or evaluation of a possible thromboembolic event.
The initial treatment should be control of the ventricular response
rate with agents that slow atrioventricular node conduction:
- β-Blockers such as intravenous esmolol (1-mg/kg by intravenous bolus)
or propranolol.
- Calcium channel blockers such as verapamil (5 to 10 mg given intravenously)
or diltiazem.[53]
If the ventricular response is excessively rapid or secondary hemodynamic instability
is present, or both, use the following guidelines:
- Synchronized direct current (DC) cardioversion starting with a relatively
high energy of 100 J and gradually increasing to 360 J is indicated.[54]
- The class III antiarrhythmic agent ibutilide (Corvert, 1 mg in 10 mL saline,
or D5
W, infused intravenously slowly over 10 minutes) has been documented
to convert atrial flutter to sinus rhythm in most patients with relatively new-onset
atrial flutter.[55]
This may be repeated once,
[56]
and although it is highly effective, life-threatening
torsade de pointes (discussed later) may occur hours after ibutilide administration,
making 4- to 8-hour monitoring after treatment highly desirable.
- Procainamide (5 to 10 mg/kg for the intravenous loading dose, infused no
faster than 0.5 mg/kg/min) may rarely be used in an attempt to restore sinus rhythm
after the ventricular response has been adequately controlled.[57]
- Treatment: Rapid atrial pacing from within
the atrium enhanced by ibutilide or procainamide also effectively terminates atrial
flutter.[58]
Atrial Fibrillation
Atrial fibrillation is an excessively rapid and irregular atrial
focus with no P waves appearing on the ECG; instead, a fine fibrillatory activity
(f waves) is seen. This is the most irregular rhythm; it is called irregularly
irregular and may be associated with a pulse deficit. The characteristics
are as follows:
- Heart rate: The atrial rate is 350 to
500 beats/min, and the ventricular rate is 60 to 170 beats/min.
- Rhythm: Irregularly irregular.
- P:QRS: The P wave is absent and is replaced
by f waves or no obvious atrial activity at all.
- QRS complex: Normal.
- Significance: The causes of atrial fibrillation
are similar to those of atrial flutter. This rhythm is often associated
with significant cardiac disease; however, idiopathic, lone paroxysmal atrial fibrillation
has become increasingly recognized. The clinical significance and treatment of atrial
fibrillation are also similar to those of atrial flutter, except for two important
considerations. The loss of an atrial "kick" from inefficient contraction of the
atria may reduce ventricular filling and may significantly compromise cardiac output.
After 24 hours, atrial fibrillation may be associated with the development of atrial
thrombi, with resultant pulmonary and systemic embolization.
- Treatment:
- Acute atrial fibrillation: The treatment
of acute atrial fibrillation is very similar to that for atrial flutter. More attention
should be focused on ventricular response, especially with the administration of
intravenous diltiazem or esmolol. Ibutilide may restore sinus rhythm, but it is
less effective than in the treatment of flutter.[55]
[56]
Synchronized DC cardioversion should be relied
on in patients with pronounced hemodynamic instability. However, if fibrillation
is present for longer than 48 hours, attempts to restore sinus rhythm may be associated
with a heightened risk of thromboembolism. In this setting for a patient with normal
coagulation function, adequate anticoagulation for 3 to 4 weeks should be considered
before attempting to restore sinus rhythm. New developments in electrophysiologic
techniques for ventricular defibrillation with biphasic current shocks have shown
superiority to the conventional monophasic current techniques (discussed later).
However, data regarding biphasic shocks for conversion of AF are still emerging.
A randomized, double-blind, multicenter trial by the BiCard Investigators[59]
demonstrated that for the cardioversion of atrial fibrillation, a biphasic shock
waveform has greater efficacy, requires fewer shocks and lower delivered energy,
and results in less dermal injury than a monophasic shock waveform. These results
mirror those seen in the ventricular fibrillation trials.
- Long-term therapy: Long-term therapy of
atrial fibrillation varies and depends on factors such as whether the arrhythmia
is constant or paroxysmal, the nature of the underlying heart disease, and the state
of ventricular function or hemodynamic stability or reserve. In the older individual
or in the setting of specific risk factors (e.g., hypertension, diabetes mellitus,
severe left ventricular systolic dysfunction), anticoagulation with warfarin should
be strongly considered. When control of ventricular response is difficult with standard
agents (e.g., β-blockers, calcium channel blockers, digitalis), electrode catheter
ablation of the atrioventricular junction and permanent pacemaker insertion have
seen increased use. This is definitive, curative, and not merely a rate-control
procedure. For patients who are in and out of atrial fibrillation, implanted atrial
defibrillators have been introduced (see Chapter
35
). They function automatically, much like the implanted ventricular
defibrillators. A detailed discussion is beyond the scope here, but a review of
their indications is available elsewhere.[60]
These
devices bypass the limitations and eliminate the side effects of current drug therapies,
which may be life threatening. In the absence of coronary artery disease or significant
left ventricular systolic dysfunction, class Ic antiarrhythmic agents (i.e., flecainide
or propafenone) have become the agents of choice.[61]
Use of class Ia drugs (i.e., quinidine, procainamide, and disopyramide) has sharply
diminished because of concerns about their significant proarrhythmic function and
their systemic and organ side effects. The use of antiarrhythmic drugs that block
repolarizing potassium currents (i.e., sotalol[62]
and amiodarone[63]
[64]
)
has gained popularity for the suppression of atrial fibrillation in individuals with
significant structural heart disease. Sotalol, however, has a much lower efficiency
of converting atrial fibrillation than class Ic agents.[65]
New class III agents show good efficacy in converting atrial fibrillation. Ibutilide
converts rapidly, can be effective in up to 50% of cases,[66]
and is more effective than sotalol or procainamide.[67]
[68]
Dofetilide, another class III agent, exhibits
a 32% conversion rate over 72 hours.[69]
All these
drugs exhibit significant proarrhythmic properties, which are summarized in Table
34-5
.[70]
Their efficacy in maintaining
sinus rhythm in patients with atrial fibrillation is summarized in Table
34-6
.
TABLE 34-5 -- Proarrhythmia with medications used to prevent the recurrence of atrial fibrillation
|
Drugs |
Cardiac Side Effects |
Comments |
|
Quinidine |
Worsening of CHF, torsades de pointes |
Most useful as a second-line agent |
|
Flecainide, propafenone |
Worsening of CHF, bradycardia, atrial and ventricular arrhythmias |
Agents of choice in lone AF; contraindicated in those with a
history of ischemia |
|
Sotalol |
Worsening of CHF, bradycardia, and torsades de pointes |
Pronounced β-blocking effects useful in those with CAD but
may exacerbate CHF |
|
Amiodarone |
Bradycardia, torsades de pointes (rare), and conduction abnormalities |
Useful in those with CHF or as a second-line agent |
|
Dofetilide |
Torsades de pointes |
Newer agent, useful in those with CHF |
|
AF, atrial fibrillation; CAD, coronary artery disease; CHF,
congestive heart failure. |
|
From Donahue TP, Conti JB: Atrial fibrillation:
Rate control versus maintenance of sinus rhythm. Curr Opin Cardiol 16:46–53,
2001. |
TABLE 34-6 -- Efficacy of antiarrhythmic medications in maintaining sinus rhythm
|
Drug |
Patients in Sinus Rhythm (%) |
Duration of Treatment (Months) |
Design of Trial |
|
Quinidine |
50 |
12 |
Meta-analysis |
|
Flecainide |
34 |
12 |
Meta-analysis |
|
Amiodarone |
60 |
12 |
Meta-analysis |
|
Sotalol |
36 |
10 |
Prospective |
|
Dofetilide |
65 |
12 |
Prospective |
|
From Donahue TP, Conti JB: Atrial fibrillation:
Rate control versus maintenance of sinus rhythm. Curr Opin Cardiol 16:46–53,
2001. |
Junctional Rhythms
The atrioventricular node itself shows no intrinsic phase 4 depolarization.
Cells in the node cannot act as pacemakers. Ectopic activity, however, may be initiated
from sites just above and below the atrioventricular node. It makes sense to consider
these arrhythmias as atrioventricular junctional in nature. The resultant P wave
is abnormal and, depending on the position of the ectopic pacemaker, may be very
close to, buried in, or after the QRS complex. Depending on the rate of fire of
the ectopic pacemaker, the resultant rhythm is nodal premature, nodal quadrigeminy,
trigeminy, or bigeminy, nodal rhythm, or nodal tachycardia.
- Heart rate: Variable, 40 to 180 beats/min
(i.e., nodal bradycardia to junctional tachycardia).
- Rhythm: Regular.
- P:QRS: 1:1 but, there are three varieties:
- High-nodal rhythm: The impulse reaches
the atrium before the ventricle; the P wave therefore precedes the QRS but has a
shortened PR interval (0.1 second).
- Mid-nodal rhythm: The impulse reaches
the atrium and the ventricle at the same time. The P wave is lost in the QRS.
- Low-nodal rhythm: The impulse reaches
the ventricle first and then the atrium, so that the P wave follows the QRS complex.
- QRS complex: Normal, unless altered by
the P wave.
- Significance: Junctional rhythms are common
in patients under anesthesia (about 20%) especially with halogenated anesthetic agents.
The junctional rhythms frequently decrease blood pressure and cardiac output by
about 15%, but they can decrease it up to 30% in patients with heart disease.[71]
- Treatment: Usually, no treatment is required,
and the rhythm reverts spontaneously. If hypotension and poor perfusion are associated
with the rhythm, treatment is indicated. Atropine, ephedrine, or isoproterenol can
be used in an effort to increase the activity of the sinoatrial node so it will take
over as the pacemaker. Amiodarone is again the drug of choice in pharmacologic therapy.
A small dose of succinylcholine (10 mg given intravenously) may revert a nodal rhythm
to a sinus rhythm during anesthesia with halothane or enflurane. This probably works
as a result of the effect of succinylcholine as a sympathetic ganglionic stimulator.
In some cases, propranolol may correct the rhythm disturbance if it is caused by
sympathetic stimulation. Dual-chamber electrical pacing at a rate faster than a
slow nodal rhythm is another option.
Ventricular Premature Beats
VPBs result from ectopic pacemaker activity arising below the
atrioventricular junction. The VPB originates in and spreads through the myocardium
or ventricular conducting system, resulting in a wide (>0.12-second), bizarre
QRS complex. The ST segment usually slopes in the direction opposite to that of
the main deflection of the QRS complex. There is no P wave associated with a VPB,
but retrograde depolarization of the atria or blocked sinus beats may obscure the
diagnosis.
The most important entity in the differential diagnosis is APB
with aberrant ventricular conduction. The distinction should be made whenever possible.
Although an APB normally reaches the sinoatrial node and resets
the sinus rhythm, such an occurrence is rare when the ectopic pacemaker is in the
ventricle. A VPB often blocks the next depolarization from the sinoatrial node,
but the following sinus beat occurs on time. The result is a compensatory pause,
consisting of the interval from the VPB to the expected normal QRS, which is blocked
at the atrioventricular node, plus a normal sinus interval.
VPBs are common during anesthesia, accounting for 15% of observed
arrhythmias. They are much more common in anesthetized patients with preexisting
cardiac disease. Other than heart disease, known etiologic factors include electrolyte
and blood gas abnormalities, drug interactions, brainstem stimulation, and trauma
to the heart.
- Heart rate: Depends on the underlying
sinus rate and frequency of the VPBs.
- Rhythm: Irregular.
- P:QRS: No P wave with the VPB.
- QRS complex: Wide and bizarre, with a
width of more than 0.12 second. If it is of an RBBB nature, prominent R forces are
present in V1
. If it is a left bundle branch block (LBBB) in appearance,
notching of the S wave and less acute downslopes are common.
- Significance: The new onset of VPBs must
be considered a life-threatening event because, in certain clinical situations, the
arrhythmia may progress to ventricular tachycardia or fibrillation. These situations
include coronary artery insufficiency, myocardial infarction, digitalis toxicity
with hypokalemia, and hypoxemia. VPBs are more likely to lead to fibrillation if
they are multiple, multifocal, or bigeminal; occur near the vulnerable period of
the preceding ventricular repolarization (i.e., R-on-T phenomenon)[72]
;
or appear in short-long-short coupling sequences.
- Treatment: The first step in treatment
is to correct any underlying abnormalities such as decreased serum potassium or low
arterial oxygen tension. If the arrhythmia is of hemodynamic significance or if
it is believed to be a harbinger of worse arrhythmias, lidocaine is usually the treatment
of choice, with an initial bolus dose of 1.5 mg/kg. Recurrent VPBs can be treated
with a lidocaine infusion of 1 to 4 mg/min; additional therapy can be supplied with
esmolol, propranolol,
procainamide, quinidine, disopyramide, atropine, verapamil, or overdrive pacing.
Ventricular Tachycardia
The presence of three or more sequential VPBs defines ventricular
tachycardia. Diagnostic criteria include the presence of fusion beats, capture beats,
and atrioventricular dissociation. The specific morphologic appearance of the QRS
complex may also be helpful in distinguishing ventricular tachycardia from other
arrhythmias. The characteristics of ventricular tachycardia are as follows:
- Heart rate: 100 to 200 beats/min.
- Rhythm: Usually regular, but may be irregular
if the ventricular tachycardia is paroxysmal.
- P:QRS: No fixed relationship because ventricular
tachycardia is a form of atrioventricular dissociation in which the P waves can be
seen marching through the QRS complex.
- QRS complex: Wide, more than 0.12 second
in width, with similar morphologic criteria in lead V1
as for VPB.
- Significance: Acute onset is life threatening
and requires immediate treatment.
- Treatment: Amiodarone administered as
one or more doses of 150 mg given intravenously in 100 mL saline or D5
W
over 10 minutes, followed by an intravenous infusion of 1 mg/min for 6 hours and
0.5 mg/min thereafter, is the recommended current treatment (maximum intravenous
dose 2.2 g/24 hours). Intravenous amiodarone has been as effective as bretylium.
[73]
Although amiodarone is associated with substantially
less hypotension compared with bretylium, hypotension and bradycardia are its main
side effects. Amiodarone's pharmacologic effects persist for more than 45 days.
Lidocaine and procainamide have been used in the past with various degrees of success
to treat ventricular tachycardia. Synchronized cardioversion is the indicated nonpharmacologic
intervention in any wide complex tachycardia, whether monomorphic ventricular tachycardia
or a wide complex supraventricular tachycardia. Polymorphic ventricular tachycardia
with a normal QT interval is treated with amiodarone and cardioversion. Metabolic
abnormalities and drug toxicity must be considered and treated. Polymorphic ventricular
tachycardia with prolonged QT interval is a more serious rhythm disturbance and the
recommended current treatment is intravenous infusion of 1 g of magnesium over 2
to 3 minutes. Precipitating metabolic or toxic causes should be treated. Overdrive
pacing may also be helpful in this setting.
Ventricular Fibrillation
Ventricular fibrillation is an irregular rhythm that results from
a rapid discharge of impulses from one or more ventricular foci or from multiple
wandering reentrant circuits in the ventricles. The ventricular contractions are
erratic and are represented on the ECG by bizarre patterns of various sizes and configurations.
P waves are not seen. Important causes of the arrhythmia include myocardial ischemia,
hypoxia, hypothermia, electric shock, electrolyte imbalance, and drug effects. The
characteristics are as follows:
- Heart rate: Rapid and grossly disorganized.
- Rhythm: Totally irregular.
- P:QRS: None seen.
- QRS complex: Not present.
- Significance: There is no effective cardiac
output, and life must be sustained by artificial means, such as external cardiac
massage.
- Treatment: Cardiopulmonary resuscitation
must be initiated immediately, and then defibrillation must be performed as rapidly
as possible. Asynchronous external defibrillation should be performed with a DC
defibrillator, using incremental energies in the range of 200 to 360 J. In extreme
situations, the use of two defibrillators in tandem across the chest, with one applied
in the anteroposterior direction and the second applied in the left midaxillary to
right midaxillary direction, has been suggested. The two devices are discharged
simultaneously to increase the likelihood of successful defibrillation. Animal studies
have failed to demonstrate the superiority of dual-pathway simultaneous shocks over
single-pathway shock.[74]
In contrast, introduction
of the biphasic (and rectilinear) transthoracic shocks has reduced the energy levels
required and increased the efficacy of ventricular defibrillation. In a prospective,
randomized, multicenter trial conducted by the ZOLL Investigators,[75]
120 joules of biphasic current were superior to 200 joules of monophasic current,
especially in patients with increased chest wall impedance.
Early administration of 1g of magnesium sulfate may facilitate
defibrillation. In some instances, epinephrine has been used to coarsen the fibrillation
just before and to facilitate defibrillation. Vasopressin has been added as a drug
for the treatment of ventricular fibrillation. The vasopressin dose is a single
40-unit intravenous bolus. If elected, subsequent administration of epinephrine
should be not earlier than 5 minutes after vasopressin. Supportive pharmacologic
therapy may include lidocaine, amiodarone, bretylium, procainamide, phenytoin, or
esmolol.
Torsade de pointes, which may mimic ventricular fibrillation or
ventricular tachycardia, is a life-threatening arrhythmia that occurs in the presence
of disturbed repolarization (hence its association with prolonged QT interval).[76]
Discontinuation of drugs that predispose to QT-interval prolongation and correction
of electrolyte abnormalities are essential in the treatment of torsades de pointes.
Acute therapy may include defibrillation, 1 to 2 g of intravenous magnesium sulfate,
intravenous amiodarone, intravenous isoproterenol, and overdrive pacing.[77]
 Figure 34-13
Sick sinus syndrome. (From Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.)
Figure 34-13
Sick sinus syndrome. (From Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.) Figure 34-14
Diagrammatic representation of various mechanisms of
supraventricular tachycardia. A-VN, A-V node; BP, bypass tract; S-AN, sinus node.
(Adapted from Marriott HJL: Practical Electrocardiography, 7th ed. Baltimore,
Williams & Wilkins, 1983.)
Figure 34-14
Diagrammatic representation of various mechanisms of
supraventricular tachycardia. A-VN, A-V node; BP, bypass tract; S-AN, sinus node.
(Adapted from Marriott HJL: Practical Electrocardiography, 7th ed. Baltimore,
Williams & Wilkins, 1983.)