Diagnosis of Ischemia
Perioperative myocardial ischemia is predictive of adverse cardiac
outcomes[78]
(see Chapter
32
and Chapter 50
).
Factors that predispose to the development of perioperative ischemia include the
presence of preexisting coronary artery disease and perioperative events that affect
the myocardial oxygen balance. Perioperative clinical studies have found a high
incidence of electrocardiographic evidence of ischemia (20% to 80%) in patients with
coronary artery disease who are undergoing cardiac or noncardiac surgery.[79]
[80]
The incidence of perioperative myocardial
infarction
in patients with coronary artery disease with or
without previous coronary artery bypass grafting (CABG) has been studied.[81]
Most patients without prior CABG in whom perioperative infarction developed had
three-vessel disease. The infarction rate in the CABG group was very low, supporting
the protective effect of prior CABG before noncardiac surgery. In the anesthetized
patient, the detection of ischemia by ECG becomes even more important because the
hallmark symptom, angina, is not available. Prolonged ischemia lasting longer than
10 minutes after vascular surgery was associated significantly with myocardial infarction
as demonstrated by serum troponin elevation.[82]
Short-term ischemic events lasting less than 10 minutes were not correlated with
postoperative infarction or cardiac complications. Prolonged ischemia was the major
cause of cardiac morbidity after major vascular surgery.
It has also become evident that a significant number of patients
suffer from asymptomatic or "silent" ischemia.[83]
Silent ischemia is manifested by characteristic electrocardiographic signs of ischemia
in the absence of angina and is not necessarily associated with changes in hemodynamics
or heart rate. Among patients with chronic stable angina who have ST-segment depression
during exercise, ambulatory electrocardiographic monitoring during daily life identifies
transient ambulant ischemic episodes in approximately 40% to 50% of patients. In
these patients, silent ischemic episodes account for about 75% of all ambulant ischemic
episodes.[84]
The electrocardiographic changes occurring during myocardial ischemia
are often characteristic and are detected with careful electrocardiographic monitoring.
Although the electrocardiographic criteria for ischemia were established in patients
undergoing exercise stress testing, they may also be applied to anesthetized patients
( Table 34-7
). These criteria
are (1) horizontal or downsloping ST-segment depression of 0.1 mV, (2) ST-segment
elevation of 0.1 mV in a non-Q wave lead, and (3) slowly upsloping ST-segment depression
of 0.2 mV (all measured from 60 to 80 msec after the J point) ( Fig.
34-15
).[85]
Okin and coworkers[86]
studied the relation of the time after the J point at which ST depression is measured
to the magnitude of ST-segment depression during peak exercise. These investigators
found that a positive exercise ECG (0.1 mV or more of additional horizontal or downsloping
ST depression at the end-exercise point) had a specificity of 96% for coronary artery
disease when ST-segment depression was measured at the J point or J + 60 msec. There
was no difference in sensitivity of electrocardiographic criteria at the J point
or at J + 60 msec. However, at J + 60 msec,
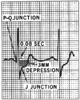 Figure 34-15
Assessment of ST-segment abnormality in the diagnosis
of ischemia. (From Ellestad MH: Stress Testing: Principles and Practice.
Philadelphia, FA Davis, 1975.)
Figure 34-15
Assessment of ST-segment abnormality in the diagnosis
of ischemia. (From Ellestad MH: Stress Testing: Principles and Practice.
Philadelphia, FA Davis, 1975.)
there were significant differences in ST-segment depression at peak exercise among
healthy persons, patients with clinical angina, and patients with documented coronary
artery disease. Investigations focused on diagnosis and monitoring for myocardial
ischemia have identified the minimum number and localization of electrocardiographic
leads for detection. V4
and V5
were identified as the most
sensitive (90% to 100% sensitivity) based on exercise stress testing[87]
[88]
and intraoperative stress ischemia monitoring.
[89]
In a perioperative study, investigators monitored 12 lead electrocardiographic
changes larger than 0.2 mV from baseline in a single lead or more than 0.1 mV in
two contiguous leads 60 msec after the J point to identify perioperative myocardial
ischemia. These changes also had to last longer than 10 minutes.[90]
Troponins were used as markers for myocardial infarction. In this study, leads
V3
and V4
were identified as most sensitive for myocardial
ischemia detection, with lead V5
trailing close behind.[90]
The sensitivity for detecting postoperative infarction was 100% for either combination
of leads V3
+ V5
or V4
+ V5
. When only a single precordial lead is available
for perioperative ischemia monitoring, as is frequently encountered, the most isoelectric
lead of V3
, V4
, or V5
should be selected. Lead
V4
rather than V5
detects ischemia earlier, is more sensitive,
and shows a greater ST-segment deviation.[90]
Lead
V4
appears to be more sensitive and appropriate for detection of prolonged
postoperative ischemia and infarction. For patients with acute coronary syndromes
(e.g., those with atherosclerotic plaque disruption), the recommendation is to monitor
limb lead III and leads V3
and V5
as the most sensitive combination
for ischemia detection.[91]
Given the debate, the
combination of an inferior limb lead along with lead V4
or V5
is prudent for ischemia detection along with good clinical care such as
adequate control of stress, heart rate, and pain for the prevention of myocardial
ischemia and infarction.[92]
It is commonly believed that monitoring for intraoperative myocardial
ischemia is unnecessary in neonates. Whereas electrocardiographic lead systems for
adults are concerned with the detection of ischemia and arrhythmias, neonatal electrocardiographic
monitoring has focused on arrhythmia recognition alone. Results of some studies,
however, suggest that the neonatal heart is more susceptible to ischemia than the
adult heart.[93]
These studies have demonstrated
the importance of calibrated electrocardiographic monitoring in neonates with congenital
heart disease (see Chapter 51
).
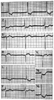 Figure 34-16
Digitalis effect on ST segments and T waves (i.e., ectopic
atrial rhythm, 2:1 atrioventricular [AV] block, type 1 second-degree AV block, left
ventricular hypertrophy with strain). (Adapted from Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.)
Figure 34-16
Digitalis effect on ST segments and T waves (i.e., ectopic
atrial rhythm, 2:1 atrioventricular [AV] block, type 1 second-degree AV block, left
ventricular hypertrophy with strain). (Adapted from Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.)
Although ST-segment analysis provides sensitive information about
myocardial ischemia, it should be remembered that underlying electrocardiographic
abnormalities hinder the analysis in about 10% of patients. These abnormalities
include hypokalemia, digitalis administration, LBBB, Wolff-Parkinson-White syndrome,
left ventricular hypertrophy with strain, and acute pericarditis ( Fig.
34-16
). In these patients, other diagnostic modalities, such as transesophageal
echocardiography, should be considered.
 Figure 34-15
Assessment of ST-segment abnormality in the diagnosis
of ischemia. (From Ellestad MH: Stress Testing: Principles and Practice.
Philadelphia, FA Davis, 1975.)
Figure 34-15
Assessment of ST-segment abnormality in the diagnosis
of ischemia. (From Ellestad MH: Stress Testing: Principles and Practice.
Philadelphia, FA Davis, 1975.) Figure 34-16
Digitalis effect on ST segments and T waves (i.e., ectopic
atrial rhythm, 2:1 atrioventricular [AV] block, type 1 second-degree AV block, left
ventricular hypertrophy with strain). (Adapted from Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.)
Figure 34-16
Digitalis effect on ST segments and T waves (i.e., ectopic
atrial rhythm, 2:1 atrioventricular [AV] block, type 1 second-degree AV block, left
ventricular hypertrophy with strain). (Adapted from Marriott HJL: Practical
Electrocardiography, 7th ed. Baltimore, Williams & Wilkins, 1983.)