 |
 |
Figure 14-9
Structural features of the Na+
channel that
determine local anesthetic (LA) interactions. A,
Consensus arrangement of the single peptide of the Na+
channel α-subunit
in a plasma membrane. Four domains with homologous sequences (D-1 through D-4) each
contain six α-helical segments that span the membrane (S1 to S6). Each domain
folds within itself to form one cylindrical bundle of segments, and these bundles
converge to form the functional channel's quaternary structure (B).
Activation gating that leads to channel opening results from primary movement of
the positively charged S4 segments in response to membrane depolarization (see panel
C). Fast inactivation of the channel follows binding
to the cytoplasmic end of the channel of part of the small loop that connects D-3
to D-4. Ions travel through an open channel along a pore defined at its narrowest
dimension by the P region formed by partial membrane penetration of the four extracellular
loops of protein connecting S5 and S6 in each domain. Intentional, directed mutations
of different amino acids on the channel indicate residues that are involved in LA
binding in the inner vestibule of the channel (X,
on S6 segments) and at the interior regions of the ion-discriminating "selectivity
filter (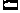 , square, on the P region), and they are also known to influence stereoselectivity
for phasic inhibition (O, also on S6 segments).
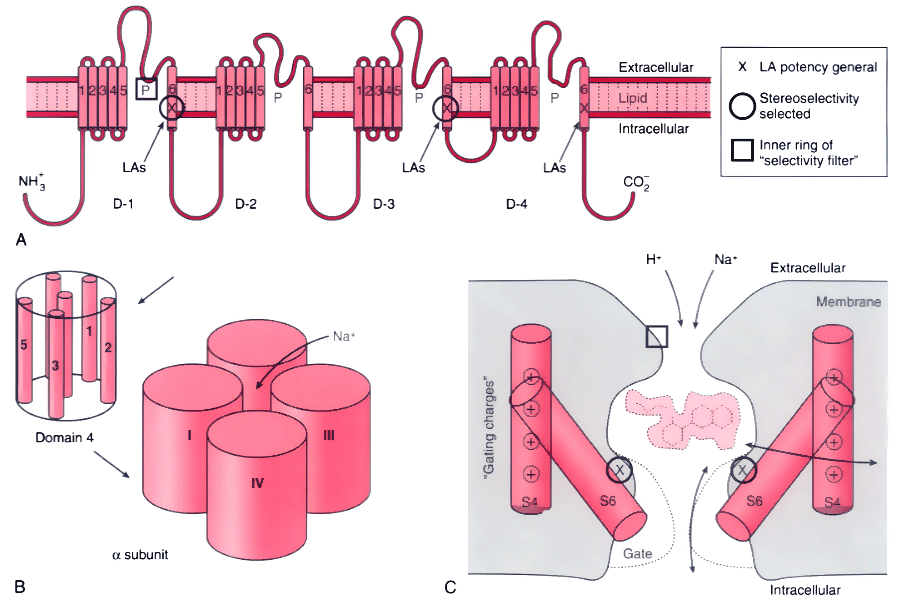
C, Schematic cross section of the channel speculating
on the manner in which S6 segments, which form a "gate," may realign during activation
to open the channel and allow entry and departure of a bupivacaine molecule by the
"hydrophilic" pathway. The closed (inactivated) channel has a more intimate association
with the LA molecule, whose favored pathway for dissociation is no longer between
S6 segments (the former pore), but now, much more slowly, laterally between segments
and then through the membrane, the "hydrophobic" pathway. Na+
ions entering
the pore will compete with the LA for a site in the channel, and H+
ions,
which pass very slowly through the pore, can enter and leave from the extracellular
opening, thereby protonating and deprotonating a bound LA molecule and thus regulating
its rate of dissociation from the channel.
, square, on the P region), and they are also known to influence stereoselectivity
for phasic inhibition (O, also on S6 segments).
C, Schematic cross section of the channel speculating
on the manner in which S6 segments, which form a "gate," may realign during activation
to open the channel and allow entry and departure of a bupivacaine molecule by the
"hydrophilic" pathway. The closed (inactivated) channel has a more intimate association
with the LA molecule, whose favored pathway for dissociation is no longer between
S6 segments (the former pore), but now, much more slowly, laterally between segments
and then through the membrane, the "hydrophobic" pathway. Na+
ions entering
the pore will compete with the LA for a site in the channel, and H+
ions,
which pass very slowly through the pore, can enter and leave from the extracellular
opening, thereby protonating and deprotonating a bound LA molecule and thus regulating
its rate of dissociation from the channel.
 |
![]() , square, on the P region), and they are also known to influence stereoselectivity
for phasic inhibition (O, also on S6 segments).
C, Schematic cross section of the channel speculating
on the manner in which S6 segments, which form a "gate," may realign during activation
to open the channel and allow entry and departure of a bupivacaine molecule by the
"hydrophilic" pathway. The closed (inactivated) channel has a more intimate association
with the LA molecule, whose favored pathway for dissociation is no longer between
S6 segments (the former pore), but now, much more slowly, laterally between segments
and then through the membrane, the "hydrophobic" pathway. Na+
ions entering
the pore will compete with the LA for a site in the channel, and H+
ions,
which pass very slowly through the pore, can enter and leave from the extracellular
opening, thereby protonating and deprotonating a bound LA molecule and thus regulating
its rate of dissociation from the channel.
, square, on the P region), and they are also known to influence stereoselectivity
for phasic inhibition (O, also on S6 segments).
C, Schematic cross section of the channel speculating
on the manner in which S6 segments, which form a "gate," may realign during activation
to open the channel and allow entry and departure of a bupivacaine molecule by the
"hydrophilic" pathway. The closed (inactivated) channel has a more intimate association
with the LA molecule, whose favored pathway for dissociation is no longer between
S6 segments (the former pore), but now, much more slowly, laterally between segments
and then through the membrane, the "hydrophobic" pathway. Na+
ions entering
the pore will compete with the LA for a site in the channel, and H+
ions,
which pass very slowly through the pore, can enter and leave from the extracellular
opening, thereby protonating and deprotonating a bound LA molecule and thus regulating
its rate of dissociation from the channel.