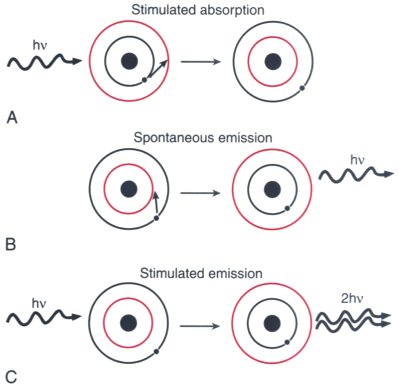
Figure 67-2
Absorption, emission, and stimulated emission. Photons
may interact with orbital electrons in three ways. A,
A photon striking an electron may transfer its energy to the electron, pushing the
electron into a higher-energy orbit. This interaction is known as absorption.
B, An electron in an orbit higher than the ground
(i.e., minimal energy) state may spontaneously lose energy in the form of an emitted
photon. C, An incoming photon may interact with an
electron that is already in a high-energy orbit, with the result that two perfectly
coherent, collimated photons leave the electron; this is known as stimulated
emission.