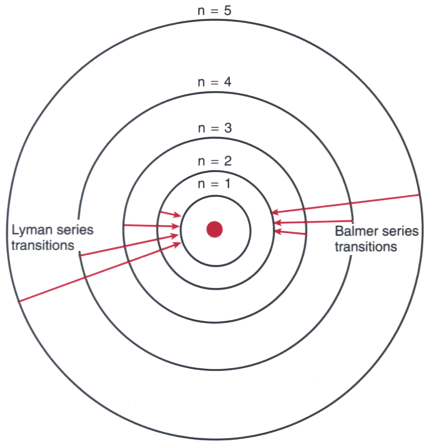
Figure 67-1
Electronic orbitals and energy levels are shown in this
schematic diagram of the Bohr model of the hydrogen atom. The circular electron
orbitals are a simplification of the probabilistic electron cloud structure. Superimposed
on the electron orbitals are the energy transitions responsible for two of the line
series in the emission spectrogram of hydrogen. The Lyman series of spectral lines
occurs when electrons release photons as they jump down to the lowest-energy orbital;
the Balmer series of lines represents jumps to an orbital just above the lowest.
The wavelength of the resulting photons is inversely proportional to the size of
the jump. For example, in the Balmer series, the jump from the n = 3 orbital to
the n = 2 orbital releases a photon of wavelength 656.3 nm, whereas the jump from
n = 5 to n = 2 is more energetic, releasing a 434-nm photon.