|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The popularity and safety of diagnostic Doppler echocardiography
in clinical medicine have driven the application of these techniques for measurement
of cardiac output. All of the ultrasound-based methods for cardiac output monitoring
use the Doppler principle. When ultrasound waves strike moving objects, these waves
are reflected back to their source at a different frequency, termed the Doppler shift
frequency, that is directly related to the velocity of the moving objects and the
angle at which the ultrasound beam strikes these objects. For blood flow measurements,
the red blood cells flowing through a major artery serve as the moving objects targeted
by the ultrasound beam. The Doppler equation describes these relationships (Equation
10). To measure blood flow velocity, this equation is rearranged to solve for velocity
(Equation 11).
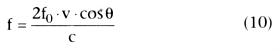

where f = Doppler shift frequency
v = velocity of red blood cell targets
f0
= transmitted ultrasound beam frequency
theta = angle between the ultrasound beam and the vector of red blood cell flow
c = velocity of ultrasound in blood (approximately 1570 m/sec)
In general, measurement of blood flow velocity requires just a single measurement, the Doppler shift frequency, because the velocity of ultrasound in blood and the transmitted ultrasound frequency are known, and cosine theta is assumed to equal 1 as long as the angle of insonation is small. This assumption requires that the ultrasound beam be oriented as much as possible in a direction that is parallel to blood flow. For example, for angles less than 20 degrees, cosine theta will be greater than 0.94, thereby introducing an error of less than 6% in the cardiac output calculation.
Once the Doppler shift frequency is measured and blood flow velocity
is calculated, stroke volume can be determined from Equation 12.
SV = v · ET · CSA (12)
where SV = stroke volume (mL)
v = spatial average velocity of blood flow (cm/sec)
ET = systolic ejection time (sec)
CSA = cross-sectional area of the vessel (cm2
)
Finally, cardiac output is derived as the product of stroke volume and heart rate.
One Doppler method that has been described for determination of cardiac output uses a PAC equipped with three 1-mm ultrasound transducers mounted on the front and rear surfaces of the catheter tip.[717] [718] [719] The theoretical advantages of PAC Doppler cardiac output monitoring are that the technique determines a space-averaged measurement of blood flow velocity in the pulmonary artery and an instantaneous measurement of vessel diameter throughout the cardiac cycle, thereby providing a form of CCO monitoring. [719] [720] However, the modest accuracy of the technique and its requirement for pulmonary artery catheterization have limited clinical acceptance.
The Doppler principle has also been applied to measure cutaneous blood flow by laser Doppler velocimetry.[721] Because this method detects only changes in skin blood flow rather than absolute cardiac output, its use is confined mainly to determining the adequacy of graft tissue flow after microvascular anastomosis of major tissue flaps.
Several different ultrasound-based methods are used to measure cardiac output, each with slightly different equipment and measuring blood flow from a different site in the body. One method involves the use of an ultrasound probe mounted on the tip of an endotracheal tube to measure transtracheal Doppler cardiac output by insonation of aortic blood flow.[722] [723] This method used pulsed-wave Doppler ultrasound to measure the flow velocity in the ascending aorta. The aortic cross-sectional area was determined by range-gating the Doppler signal in small 1-mm increments to measure aortic diameter and then calculating the aortic cross-sectional area, assuming the aorta to be a circular vessel.[723] Although early reports suggested that this method tracked directional changes in cardiac output accurately, Hausen and coauthors reported very poor performance of the device in the postoperative setting, with transtracheal Doppler measurements markedly underestimating thermodilution cardiac output values.[724] Practical considerations have also been unfavorable, including marked operator dependency for success because of a difficult user interface and poor signal stability.[725] This method required tracheal intubation and patient sedation, which further limited its applicability. When the Doppler signal is inadequate, the endotracheal tube cuff needed to be deflated and the tube repositioned, thus placing the patient at risk for pulmonary aspiration.[724] As a result of these considerations, transtracheal Doppler cardiac output measurement has not been widely accepted in clinical practice.
Other clinical methods to measure cardiac output with Doppler ultrasound have focused on less invasive approaches than the transtracheal or PAC techniques. One method was developed to measure blood flow velocity in the distal aortic arch with an ultrasound transducer applied to the suprasternal notch.[726] Further refinements of the technique used continuous-wave Doppler to measure the systolic velocity integral and estimate stroke volume in the ascending aorta.[727] By the 1980s, successful noninvasive measurement of cardiac output was reported involving the use of commercially available equipment to determine velocities in the ascending aorta with continuous-wave Doppler and the aortic cross-sectional area with A-mode pulsed echocardiography.[728] [729] [730] Although these suprasternal Doppler cardiac output techniques showed reasonable agreement with thermodilution methods, they were relatively labor intensive, required a fair degree of experience and expertise, and could not be accomplished in a significant number of critically ill medical and surgical patients.[728] [730] [731] Because they provided only intermittent measurements at best, clinical acceptance of this method was limited.
The evolution of Doppler techniques into practical clinical monitoring tools required an ultrasound transducer that could be positioned and left in place for continuous monitoring without the need for repeated adjustments or time-consuming measurements by the physician. Such a transducer was incorporated into the tip of a standard esophageal stethoscope to allow continuous monitoring of cardiac output by interrogating the blood flow profile in the descending thoracic aorta. This technique, esophageal Doppler cardiac output monitoring, is one of the more widely applied methods for noninvasive or minimally invasive monitoring of cardiac output.[626] In brief, the Doppler probe is inserted into the esophagus to a depth of approximately 35 cm from the incisors in a tracheally intubated patient. Probe position is adjusted to optimize the audible Doppler flow sound from the descending aorta. In most patients, optimal probe tip position is at the T5-6 vertebral interspace or the third sternocostal junction because the esophagus and the descending aorta lie in close proximity and run essentially parallel to one another at this location.[732] [733] [734] The ultrasound transducer is mounted at a fixed angle that is known by the cardiac output computer and used to correct the resulting Doppler shift frequency to provide an accurate velocity measurement (see earlier discussion).
The esophageal Doppler monitoring method interrogates blood flow in the descending thoracic aorta and therefore measures only a fraction of total cardiac output. Consequently, the esophageal Doppler probe must be "calibrated" by some method. Most early investigators performed this initial calibration with a suprasternal Doppler measurement of cardiac output in the ascending aorta.[729] [735] [736] [737] [738] [739] In some instances, ascending aortic diameter was also measured by A-mode ultrasound,[729] [735] whereas later versions of these monitors used a nomogram to estimate aortic diameter based on the patient's age, sex, height, and weight.[736] [737] [738] [739]
The initial studies comparing cardiac output measured with the esophageal Doppler device with other methods showed variable performance of the new technique.[740] [741] Although several investigators demonstrated accurate tracking of changes in cardiac output measured by thermodilution, there were significant differences in absolute cardiac output between methods, with the Doppler technique both underestimating and overestimating simultaneous thermodilution values.[729] [736] Other investigations were even less favorable, thus suggesting that the esophageal Doppler method could not be relied on as a trend monitor.[738] [739] [742] It appeared that the suprasternal Doppler measurements and the aortic diameter measurements were often in error and did not provide suitable initial calibration of the esophageal Doppler probe. Furthermore, these initial calibration methods were
In recent years, the esophageal Doppler method for cardiac output monitoring has seen renewed enthusiasm, not so much as an accurate measure of absolute cardiac output, but as an estimate for systemic blood flow.[626] [743] Singer and colleagues have popularized this technique in the United Kingdom by applying it to the care of critically ill patients and using newer features of the Doppler technique to discern additional hemodynamic information.[744] [745] [746] [747] Improvements in instrument design have made it more informative to the clinician and much simpler to use. Current devices provide a clear visual display of the spectral Doppler waveform and calculate and display additional hemodynamic variables, including the peak blood flow velocity, flow acceleration, and the heart rate-corrected flow time. Some studies have shown that these additional measures provide useful information about left ventricular preload, contractility, and SVR.[626] [744] [745] [746] [747] [748] [749] Esophageal Doppler monitoring provides a continuous, beat-to-beat waveform that is proportional to left ventricular stroke volume. One of the more important values of the monitor may be that it focuses clinical attention on optimizing stroke volume rather than total cardiac output. Indeed, in critically ill patients, complications may be predicted better by low stroke volume than by low cardiac output.[750]
The newer esophageal Doppler monitoring devices are simpler to operate because they do not require the cumbersome calibration procedures demanded by earlier devices. The emphasis in developing these devices has been more toward providing a continuous measure of aortic blood flow rather than total cardiac output. [751] [752] For the Doppler technique to provide accurate trending information, however, several assumptions must still remain valid. First, the angle between the esophageal Doppler probe and the descending aorta must be known and remain constant throughout the monitoring period. Second, the aortic cross-sectional area must remain constant. Third, the descending aortic flow must remain a constant proportion of the total cardiac output. [753]
The esophageal Doppler monitoring technique has a number of attributes that have aided its clinical acceptance. The technique is easy to use, minimally invasive, and inherently safe. It has been used successfully for days in tracheally intubated patients or sedated patients in the intensive care unit.[734] This monitoring can be initiated rapidly, usually within 5 minutes, and can be applied when pulmonary artery catheterization cannot be accomplished or is contraindicated. [754] It appears that limited experience is needed for clinical success—as few as 10 to 12 cases for accurate application of the technique.[734] A recent review of 25 clinical trials comparing esophageal Doppler cardiac output measurement with PAC thermodilution measurement noted that the Doppler cardiac output values correlated well with thermodilution measurements, showed minimal overall bias and good tracking of directional changes in thermodilution cardiac output, and had low intraobserver and inter-observer measurement variability.[734]
The main limitation of esophageal Doppler cardiac output monitoring remains its accuracy as an absolute measure of cardiac output. In an attempt to address this problem, the latest modifications of these devices have focused on direct measurement of aortic diameter. These newer monitors use esophageal Doppler probes containing two ultrasound transducers. One is used to measure aortic blood flow by pulsed-wave Doppler, and the second measures aortic diameter simultaneously at the flow site by M-mode echocardiography.[732] [733] These monitors display both the Doppler and the M-mode waveforms to promote user confidence in the quality of these signals. The advantage of the added aortic imaging data is that assumptions regarding aortic diameter are not needed and, instead, are replaced by continual aortic measurements. Whether these newer monitors will provide more accurate cardiac output values than possible with the simpler Doppler technologies remains uncertain.
Other limitations of the esophageal Doppler technique must also be appreciated by the physician to avoid pitfalls in interpretation of data. First and foremost, as emphasized earlier, the technique provides only an estimate of total cardiac output. Furthermore, for changes in cardiac output to be properly detected, the previously mentioned assumptions regarding descending aortic blood flow must remain valid. The technique is likely to be inaccurate in patients with aortic valve stenosis or regurgitation or those with thoracic aortic disease. Moreover, a fundamental assumption that descending aortic blood flow is approximately 70% of total cardiac output is not valid in the presence of conditions that redistribute blood flow, such as pregnancy, aortic cross-clamping, and after cardiopulmonary bypass.[729] [734] [737] The esophageal Doppler technique is not easily applied in non-tracheally intubated patients, and it cannot be used in individuals with esophageal pathology. Finally, like all ultrasound techniques, the acoustic window to acquire the Doppler signal may not be adequate in some individuals, thereby precluding use of this method.
The current clinical role for esophageal Doppler cardiac output monitoring is not to serve as a replacement for the PAC, but rather as an additional circulatory monitor in higher-risk patients who do not warrant invasive monitoring. [626] [755] The popularity of this technique in the United Kingdom may reflect in part the much more restricted use of PACs in that country versus the United States. Preliminary studies have suggested that perioperative esophageal Doppler-guided volume resuscitation of moderate-risk surgical patients reduces perioperative morbidity and shortens the hospital stay.[756] [757] If these results can be duplicated in other settings and with larger groups of patients, this technique may find a role as a more routine monitor in patients who are at increased risk for perioperative circulatory complications.
|
|
|
|
|
|
|
|
|
|
|
|
|