Other Measures of Anesthetic Depth
Evoked Responses
Sensory or nerve stimulation produces a low-amplitude signal,
or evoked response, within the CNS. This evoked response can be separated by means
of special computer signal-averaging techniques from the underlying, spontaneous
EEG. The ability to evoke a response is a measure
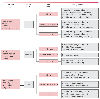 Figure 31-23
Strategy for titrating hypnotic and opioid based on integration
of the bispectral (BIS) index value with observed clinical response. (Redrawn
from Kelley SD: Monitoring Level of Consciousness during Anesthesia and Sedation.
Natick, MA, Aspect Medical Systems, 2003.)
Figure 31-23
Strategy for titrating hypnotic and opioid based on integration
of the bispectral (BIS) index value with observed clinical response. (Redrawn
from Kelley SD: Monitoring Level of Consciousness during Anesthesia and Sedation.
Natick, MA, Aspect Medical Systems, 2003.)
of the functional integrity of the sensory receptors and the pathways between the
sensory receptor and neural generator of peaks in the evoked response waveform.
Evoked responses are used primarily to monitor the functional integrity of the neural
structures, to identify neural structures, and to diagnose neurophysiologic conditions.
Because evoked responses are sensitive to anesthetic drugs, they have been investigated
as possible measures of anesthetic drug effect and depth of anesthesia.[139]
The sensory stimulation most commonly used in recording of evoked responses is somatosensory
(electrical) stimulation of peripheral nerves, auditory stimulation using clicks
at the auditory canal, visual stimulation using flashing lights, or electrical stimulation
of tooth pulp.
Recording of evoked responses involves recording EEG epochs synchronized
to the repetitive sensory stimuli.
Computer techniques for processing EEG signals extract the evoked potential from
the underlying EEG. Typically, the algorithm requires 100 to 1000 stimuli to extract
the evoked potential from the underlying EEG signal. Therefore, the evoked response
represents a time-versusvoltage relationship that can be quantitated by measuring
the poststimulus latency and interpeak amplitudes in the waveform. Evoked response
methodology has been reviewed by Grundy.[186]
[187]
Many investigations have been performed with evoked potentials,
with special emphasis on midlatency auditory evoked potentials (MLAEPs).[188]
[189]
[190]
[191]
[192]
[193]
[194]
[195]
MLAEPs have been shown to be significantly
affected by anesthetic hypnotic drugs in a graded, reversible, and nonspecific manner.
Specifically, the amplitudes (microvolts) and the latency (milliseconds) of the
waves Pa and Nb have been examined. Hypnotic anesthetic drugs decrease the amplitudes
and increase the latencies of these two waves. Opioids in clinically relevant concentrations
produce minimal changes in MLAEPs.[196]
Iselin-Chaves and colleagues[197]
compared the MLAEP and BIS index in volunteers receiving stepped increases in propofol
concentrations or propofol and alfentanil while recording clinical sedation scores.
They found that both the BIS and MLAEP patterns showed significant changes with
increasing levels of sedation, with the Pa and Nb waves decreasing in amplitude and
increasing in latency. The BIS index correlated better with clinical sedation scores
relative to the MLAEPs; however, MLAEPs showed the best correlation with plasma drug
concentrations. The addition of 100 ng/mL alfentanil did not affect the relationship
between MLAEP and loss of consciousness.
MLAEPs have inconveniences that to date have limited their clinical
use.[197]
These limitations include (1) considerable
time needed to produce a response, 0.5 to 5 minutes; (2) complex setup, with MLAEPs
typically requiring more than 5 minutes of patient preparation; (3) need for intact
hearing; and (4) lack of a univariate parameter calibrated to the anesthetic state.
Mantzaridis and Kenny addressed some of these limitations by introducing a new MLAEP
parameter, the auditory evoked potential (AEP) index, based on a proprietary algorithm.
[198]
The AEP simplifies interpretation of the
MLAEP
waveforms, but it still requires significant time for the signal-averaging process.
Jensen and coworkers developed a new adaptive method for extracting the MLAEP from
the EEG signal that involves an autoregressive model with an exogenous input (ARX)
to allow extraction of the AEP signal within 15 to 25 sweeps of 110-msec duration;
the process results in only a 6-second delay.[199]
This concept has been incorporated into a commercially available device that calculates
the A-Line ARX Index (AAI) from the fast-extracted MLAEP waveform analysis (A-Line
Monitor, Danmeter A/S, Odense, Denmark). The AAI, like the BIS index, ranges from
100 (awake) to 0 (deep hypnotic effect). This variable represents the only commercially
available device analogous to the BIS monitor, but it uses AEPs. Struys and colleagues
[200]
compared this autoregressive modeling with
exogenous input of MLAEPs to the BIS index in patients receiving propofol. Target
effect-site concentrations were achieved and progressively increased in steps with
clinical measurement of the level of sedation (OAA/S scores) and defined noxious
stimuli. Both the BIS index and the AAI were accurate indicators of the level of
sedation and loss of consciousness. With clinical stimuli, greater variability was
seen with the AAI than with the BIS index. The BIS index correlated best with propofol
effect-site concentrations. Neither methodology predicted reaction to noxious stimuli.
Hemodynamic variables were poor indicators of the hypnotic state.
One recent study compared the AAI with the BIS index in surgical
patients undergoing transitions from wakefulness to unconsciousness.[201]
The authors concluded that the BIS index and AAI were superior to hemodynamic variables
and classic single-parameter EEG variables (i.e., median frequency). The BIS index
provided better discrimination between unconscious and awake states than the AAI
index did.
 Figure 31-23
Strategy for titrating hypnotic and opioid based on integration
of the bispectral (BIS) index value with observed clinical response. (Redrawn
from Kelley SD: Monitoring Level of Consciousness during Anesthesia and Sedation.
Natick, MA, Aspect Medical Systems, 2003.)
Figure 31-23
Strategy for titrating hypnotic and opioid based on integration
of the bispectral (BIS) index value with observed clinical response. (Redrawn
from Kelley SD: Monitoring Level of Consciousness during Anesthesia and Sedation.
Natick, MA, Aspect Medical Systems, 2003.)