|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The purposeful movement of a body part in response to noxious perioperative stimuli has been extensively used as a clinical sign of anesthesia. Using this movement to quantitate anesthetic response induced by potent inhaled anesthetics, Eger and Merkel and their colleagues[13] [78] defined MAC as the minimum alveolar concentration of inhaled anesthetic required to prevent 50% of subjects from responding to a painful stimulus with "gross purposeful movement." Readers are referred to excellent review articles that document development of the MAC concept and its many applications in anesthesia.[79] [80] The MAC concept has four basic components: (1) an all-or-none (quantal) movement response must occur after the application of a supramaximal noxious stimulus; (2) end-tidal concentrations of anesthetic in the alveoli, considered an equilibrated sample site, are used as an indication of the concentration of anesthetic in the brain; (3) appropriate mathematical quantitation of the relationship between the alveolar concentration of anesthetic and the quantal response is used to estimate MAC; and (4) MAC can be quantitated for altered physiologic and pharmacologic states.
For determination of MAC in humans, the standard noxious stimulus has been the initial surgical skin incision.[13] Skin incision represents a reproducible form of supramaximal surgical stimulation. There has been no systematic examination of other perioperative surgical stimuli (e.g., peritoneal traction) representing more profound surgical manipulation than skin incision or endotracheal intubation. For determination of MAC in animals, the standard stimulus has been the application of a surgical clamp to the base of the tail. After examining other noxious stimuli in dogs, Eger and colleagues[13] concluded that tail clamping represented the most noxious stimulation that was clinically reproducible and not excessively traumatic. The MAC concept has been expanded by evaluating other clinical end points and defined stimuli. Stoelting and associates [81] determined the MAC of anesthetic that would allow opening of the eyes on verbal command during emergence from anesthesia ("MACawake "). This stimulation is less intense than surgical skin incision, and response occurs at lower concentrations of anesthetic than is the case with movement to skin incision. Generally, MACawake values are a third to a fourth the MAC values for surgical incision. Yakaitis and coworkers[82] determined the MAC of inhaled anesthetic that would inhibit movement and coughing during endotracheal intubation ("MACintubation "). Intubation is significantly more stimulating than skin incision, and higher concentrations of inhaled anesthetic are required to eliminate the movement response. Finally, Roizen and associates[83] investigated the MAC of anesthetic necessary to prevent an adrenergic response to skin incision ("MACBAR "), as measured by the concentration of catecholamine in venous blood. When one examines the values for (1) MACawake , (2) MACskin incision , (3) MACintubation , and (4) MACBAR , one sees a family of concentration-versus-response curves that characterize the hypnotic effects of inhaled anesthetics relative to defined clinical stimuli.
Zbinden and colleagues[84] undertook a comprehensive examination of the effects of different noxious stimuli on the purposeful movement response with isoflurane. Twenty-six healthy surgical patients were administered isoflurane only with adequate inspired/end-tidal equilibration. Multiple noxious stimuli were applied at varying end-tidal isoflurane concentrations. From the resulting data, the authors were able to define a family of concentration-versus-response curves for different noxious stimuli, as displayed in Figure 31-11 . The end-tidal isoflurane concentrations that resulted in a 50% probability of no movement response to different stimuli were as follows: verbal responsiveness, 0.37%; trapezius muscle squeeze, 0.84%; laryngoscopy, 1.0%; 50-Hz electrical tetanus, 1.03%; skin incision, 1.16%; and laryngoscopy with intubation, 1.76%. This study demonstrates how varying clinical stimuli require different isoflurane concentrations to prevent a clinical response and can be used to define a concentration-versus-response relationship for the hypnotic effects of isoflurane.
A second component of the MAC concept involves use of the alveolar concentration of an anesthetic as an indication of drug concentration. Because the concentration
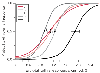 Figure 31-11
Logistic regression analysis of the end-tidal isoflurane
concentration relative to the predicted probability of no movement response for different
noxious stimuli. Bars indicate the 95% confidence
bounds to the end-tidal concentration with a 50% probability of response. I, laryngoscopy/intubation;
L, laryngoscopy; S, trapezius muscle squeeze; SI, skin incision; T, tetanic nerve
stimulation. (Redrawn with modification from Zbinden AM, Maggiorini M, Petersen-Felix
S, et al: Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen
anesthesia. I. Motor reactions. Anesthesiology 80:253, 1994.)
Figure 31-11
Logistic regression analysis of the end-tidal isoflurane
concentration relative to the predicted probability of no movement response for different
noxious stimuli. Bars indicate the 95% confidence
bounds to the end-tidal concentration with a 50% probability of response. I, laryngoscopy/intubation;
L, laryngoscopy; S, trapezius muscle squeeze; SI, skin incision; T, tetanic nerve
stimulation. (Redrawn with modification from Zbinden AM, Maggiorini M, Petersen-Felix
S, et al: Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen
anesthesia. I. Motor reactions. Anesthesiology 80:253, 1994.)
 Figure 31-12
Minimal alveolar concentration necessary to prevent movement
in 50% of subjects receiving a noxious stimulus for halothane combined with (1) oxygen,
(2) oxygen and morphine premedication (0.15 mg/kg intramuscularly), and (3) 70% nitrous
oxide. The anesthetic requirement for halothane is greatly decreased by nitrous
oxide and less so by premedication with morphine. (Modified from Saidman
LJ, Eger EI II: Effect of nitrous oxide and of narcotic premedication on the alveolar
concentration of halothane required for anesthesia. Anesthesiology 25:302, 1964.)
Figure 31-12
Minimal alveolar concentration necessary to prevent movement
in 50% of subjects receiving a noxious stimulus for halothane combined with (1) oxygen,
(2) oxygen and morphine premedication (0.15 mg/kg intramuscularly), and (3) 70% nitrous
oxide. The anesthetic requirement for halothane is greatly decreased by nitrous
oxide and less so by premedication with morphine. (Modified from Saidman
LJ, Eger EI II: Effect of nitrous oxide and of narcotic premedication on the alveolar
concentration of halothane required for anesthesia. Anesthesiology 25:302, 1964.)
A third component of the MAC concept involves the use of appropriate mathematical approaches to quantitate the relationship between dose and response. The original MAC concept of Eger and colleagues[13] used a "bracketing approach" in humans and animals. In an individual patient, a fixed end-tidal concentration of anesthetic was achieved, and the response to a single skin incision was observed. Depending on the patient's response or lack of response, the next patient received a higher or lower concentration. A single measurement was obtained per patient. Patients were studied over a range of end-tidal concentrations. They were placed in groups of four; the subject having the lowest end-tidal (alveolar) concentration of anesthetic was the first to be studied. For each group, the percentage of patients moving in response to stimulation was plotted against the average end-tidal concentration for that group. A visual line of "best fit" through these points yielded the concentration at which 50% of patients would respond (i.e., the MAC). An example of such analysis is presented in Figure 31-12 , which uses the concentration-versus-response data gathered for halothane alone, for halothane with morphine premedication, and for halothane with 70% nitrous oxide. Both morphine premedication and 70% nitrous oxide decreased the MAC value for halothane.[86]
In animal studies, it was possible to manipulate the end-tidal concentration of anesthetic and apply the tail clamp stimuli on multiple occasions. A MAC value can be obtained for each animal by sequentially increasing or decreasing the end-tidal concentrations to bracket the value between movement and no movement. De Jong and Eger[87]
A fourth feature of MAC is that it has served as a sensitive tool to determine the interaction of other anesthetics and CNS drugs with the inhaled anesthetics. Other drugs used in anesthesia decrease anesthetic requirements, as measured by a reduction in MAC. In addition, numerous altered physiologic states (e.g., aging) change the requirements for inhaled anesthetics. Table 31-2 summarizes the results of studies regarding factors that affect MAC.
Much of the previous research on MAC assumed that the lack of movement response with inhaled anesthetics was due to anesthetic effects on the central, cortical brain tissues. This assumption has been challenged. In 1993, Rampil and colleagues demonstrated that the spinal cord represents a major site of action for inhaled anesthetics.[29] Their studies showed that the MAC of isoflurane in rodents was identical in value for intact animals and rodents that were decorticate or decerebrate, a finding suggesting that the main ability of inhaled anesthetics in preventing purposeful movement occurs at the spinal cord level.[29] [30] Antognini and Schwartz also drew the same conclusions from experiments whereby they determined MAC in the goat by having the animal's brain isolated from the remainder of the body through a complex cardiopulmonary bypass procedure.[31] These investigators demonstrated that the MAC value for the goat was approximately twice as large when only the brain was exposed to isoflurane as opposed to both the brain and the spinal cord. These series of studies demonstrate that the response to purposeful movement is achieved by inhaled anesthetics at subcortical anatomic levels, on the spinal cord. Eger and associates proposed that volatile anesthetics cause a lack of movement response to noxious stimuli by action in the spinal cord and create a hypnotic/amnestic loss of consciousness at a supraspinal, cortical site of action.[90]
Responses other than purposeful movement have been investigated
as possible clinical measures of the depth of anesthesia: the rate and volume of
ventilation in
| Effect on MAC | Factors (Study Subjects) |
|---|---|
| Decrease | Hypothermia (animals) |
|
|
Severe hypotension (animals) |
|
|
Age (humans) |
|
|
Opioids, ketamine (humans, animals) |
|
|
Chronic administration of amphetamine (animals) |
|
|
Reserpine, α-methyldopa (animals) |
|
|
Cholinesterase inhibitors (animals) |
|
|
Intravenous local anesthetics (humans, animals) |
|
|
Pregnancy (animals) |
|
|
Hypoxemia (PaO2 <40 mm Hg) (animals) |
|
|
Anemia (animals) |
|
|
α2 -Agonists (animals, humans) |
| Increase | Hyperthermia (animals) |
|
|
Hyperthyroidism (animals) |
|
|
Alcoholism (humans) |
|
|
Acute administration of dextroamphetamine (animals) |
| No effect | Duration of anesthesia (humans, animals) |
|
|
Sex (human, animals) |
|
|
Metabolic acid-base status (animals) |
|
|
Hypercapnia and hypocapnia (humans, animals) |
|
|
Isovolemic anemia (animals) |
|
|
Hypertension (animals) |
| Adapted from Quasha AL, Eger EI II, Tinker JH: Determination and applications of MAC. Anesthesiology 53:315, 1980. | |
Zbinden and colleagues[92] systematically examined the interaction of isoflurane concentrations with the hemodynamic response to different noxious stimuli. In 26 healthy surgical patients receiving different equilibrated end-tidal concentrations of isoflurane, the following noxious stimuli were applied, several of them on multiple occasions: trapezius muscle squeeze, 50-Hz electrical tetanus, laryngoscopy, laryngoscopy with intubation, and skin incision. With continuous recording of intra-arterial hemodynamics, acute increases in heart rate and systolic blood pressure were noted and related to the isoflurane concentration and the presence or absence of purposeful movement. Table 31-3 presents the absolute increase in systolic blood pressure and heart rate at the isoflurane end-tidal
| Stimulus | End-Tidal Concentration That Suppresses Movement in 50% of Patients (ET50 ) (Percent atm) | Change in Systolic Blood Pressure for Patients at ET50 (mm Hg) | Change in Heart Rate for Patients at ET50 (Beats/min) |
|---|---|---|---|
| Trapezius muscle squeeze | 0.90 | 9 | 5 |
| Electrical tetanus | 1.10 | 15 | 15 |
| Laryngoscopy | 1.07 | 23 | 17 |
| Skin incision | 1.24 | 34 | 35 |
| Laryngoscopy and intubation | 1.87 | 53 | 36 |
| Adapted from Zbinden AM, Petersen-Felix S, Thomson DA: Anesthetic depth defined using multiple noxious stimuli during isoflurane/oxygen anesthesia. II. Hemodynamic responses. Anesthesiology 80:261, 1994. | |||
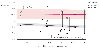 Figure 31-13
Response of systolic blood pressure (BP, mm Hg) to skin
incision as a function of end-tidal isoflurane concentration. Filled
squares, systolic blood pressure before stimulation; open
squares, systolic blood pressure after stimulation; lower
oblique line, regression line to systolic blood pressure before stimulation
(r = -.46); upper line,
regression line to systolic blood pressure after skin incision (r
= .0002); shaded area, 95% confidence interval for
regression lines; vertical arrow, median effective
isoflurane concentration for the motor response of the stimulation pattern. (Redrawn
from Zbinden AM, Petersen-Felix S, Thomson DA: Anesthetic depth defined using multiple
noxious stimuli during isoflurane/oxygen anesthesia. II. Hemodynamic responses.
Anesthsiology 80:261, 1994.)
Figure 31-13
Response of systolic blood pressure (BP, mm Hg) to skin
incision as a function of end-tidal isoflurane concentration. Filled
squares, systolic blood pressure before stimulation; open
squares, systolic blood pressure after stimulation; lower
oblique line, regression line to systolic blood pressure before stimulation
(r = -.46); upper line,
regression line to systolic blood pressure after skin incision (r
= .0002); shaded area, 95% confidence interval for
regression lines; vertical arrow, median effective
isoflurane concentration for the motor response of the stimulation pattern. (Redrawn
from Zbinden AM, Petersen-Felix S, Thomson DA: Anesthetic depth defined using multiple
noxious stimuli during isoflurane/oxygen anesthesia. II. Hemodynamic responses.
Anesthsiology 80:261, 1994.)
The clinical implications of these data relative to judging the anesthetic depth of inhaled anesthetics from the hemodynamic response are significant. When used as a sole agent, even at high concentrations isoflurane is unable to suppress hemodynamic responses to noxious stimuli. Instead, the hemodynamic control seen with high isoflurane concentrations occurs as a result of a decrease in the prestimulation hemodynamic baseline value. Thus, although hemodynamic responses are the most commonly used clinical measures to judge the inhaled anesthetic depth of anesthesia, the scientific basis for this is certainly not obvious. This study is important evidence that isoflurane is providing hypnotic anesthetic effects with minimal analgesic effect, as judged by the profound hemodynamic responses that occurred with noxious stimuli. In clinical practice, additional anesthetic drugs are commonly used with inhaled anesthetics. Daniel and colleagues[37] examined how fentanyl (0 to 3 µg/kg) and 60% nitrous oxide alter the heart rate, mean arterial blood pressure, and catecholamine response (components of MACBAR ) during desflurane and isoflurane anesthesia.
|
|
|
|
|
|
|
|
|
|
|
|
|