|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disorders of calcium, magnesium, and phosphate balance were discussed in the section on diseases involving the endocrine system and disorders of nutrition.
Electrolyte disorders are usually detected by determining the
levels of electrolytes in serum. These concentrations reflect the balance between
water and electrolytes. The osmolality of all body fluids is normally maintained
within the narrow physiologic range of 285 to 290 mOsm/kg H2
O by integration
of three key processes: thirst, release of ADH, and responsiveness of the medullary
collecting ducts to ADH. Because of the permeability of biologic membranes, intracellular
osmolality and extracellular osmolality are almost always equal and can be estimated
by the following formula:
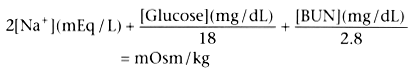
This formula will become easier to calculate when we convert fully to the Système
International d'Unités (metric system) because millimoles (mmol) can be substituted
for mg/(factor) in the above formula to read
2[Na+
] + [Glucose] + [BUN] =
mOsm/kg
with concentrations expressed in millimoles per liter (mmol/L). Although secretion
of ADH is tightly controlled by osmotic stimuli at 285 to 290 mOsm/kg, the osmotic
threshold for thirst is high (300 mOsm/kg), thus making this sign an important guide
to volume deficiency.
Hyponatremia is perhaps the third most common fluid-electrolyte abnormality in hospitalized patients. (Magnesium deficiency occurs in as many as 25% [see Chapter 25 ] and potassium deficiency, discussed later in this section, in as many as 10%.) Hyponatremia can occur
The more common isovolumic hypotonic hyponatremia is caused by retention of water without sodium. Because edema is not usually clinically apparent, such patients appear isovolumic. Edema is most often caused by SIADH, which in turn may be caused by CNS or pulmonary tumors or dysfunction. Secretion of ADH increases with age, thus rendering the elderly more prone to hyponatremia. Drugs that potentiate the secretion of ADH (tricyclic antidepressants and vincristine) or its effects on the medullary collecting duct system in the kidney (nonsteroidal antiinflammatory drugs and chlorpropamide) or that have similar effects (oxytocin) may be more likely to cause hyponatremia in the elderly. To establish the diagnosis of SIADH, the physician should determine that the patient is free of renal and cardiac dysfunction, has normal adrenal and thyroid function, and is normovolemic. Urine osmolality would then be found to exceed 100 mOsm/kg, serum osmolality would be low, and urine sodium excretion would be higher than 20 mEq/L (20 mOsm/L).
Disturbances in serum sodium therefore reflect alterations in glucose metabolism, renal function, or accumulation of body water. The last can be affected by disturbances in thirst, release of ADH, and renal function. Thus, hyponatremia reflects a relative excess of free water and can occur when total-body sodium increases (as in edematous disorders), when total-body sodium is normal (as in excess of free water because of SIADH), or when total-body sodium decreases (as occurs with too aggressive use of diuretic drugs). Definition of the cause defines the treatment. For instance, water restriction is the mainstay of therapy for SIADH. Administration of demeclocycline is another option that corrects SIADH by inducing a reversible nephrogenic diabetes insipidus. The anesthesiologist is faced with the question of what levels of electrolytes require treatment before anesthesia. Although slowly developing hyponatremia usually produces few symptoms, the patient may be lethargic and apathetic. Chronic hyponatremia is better tolerated than acute hyponatremia because of mechanisms regulating intracellular fluid volume that alleviate brain edema; the loss of other solutes from cells decreases the osmotic movement of water into cells. Nonetheless, severe chronic hyponatremia (i.e., serum sodium levels <123 mEq/L) can cause brain edema.[761] [762] By contrast, acute hyponatremia may be manifested by severe symptoms requiring emergency treatment: profound cerebral edema with obtundation, coma, convulsions, and disordered reflexes and thermoregulatory control.[761] [762] Depending on the cause and relative total sodium and water content, treatment can range from administration of hypertonic saline or mannitol (with or without diuretic drugs) to restriction of fluids or administration of other drugs.[280] [761] [762] Because neurologic damage may develop if the serum sodium concentration is increased too rapidly, the rate of increase should not exceed 1 mEq/L/hr.[279] [280] [762] After the serum sodium concentration has reached 125 mEq/L, therapy may consist of water restriction; more rapid correction may result in CNS demyelination.[279] [280] [762] In hyponatremic patients who have excess total-body water secondary to SIADH, serum levels can be corrected by giving furosemide, 1 mg/kg, and hypertonic saline to replace the loss of electrolytes in urine.[279] [761] [762] The diagnosis of SIADH is discussed earlier in this chapter (see the section "Pituitary Abnormalities").
Neither acute nor chronic hyponatremia necessitates the restoration of serum sodium to normal levels; brain swelling usually disappears at a serum sodium level of 130 mEq/L. This leaves us with the question of what levels of serum sodium make anesthesia more risky. Because no data exist to answer this question, to allow for some error in caring for patients, we have arbitrarily chosen a flexible concentration of 131 mEq/L as the lower sodium limit for elective surgery. A discussion of intraoperative hyponatremia in patients undergoing transurethral prostatectomy[758] can be found in Chapter 54 .
Hypernatremia occurs much less commonly than hyponatremia. It is often iatrogenic in origin (e.g., it can be caused by failure to provide sufficient free water to a patient who is unconscious or who has had a recent stroke-induced deficit of the thirst mechanism) and can occur in the presence of low, normal, or excess total-body sodium. The primary symptoms of hypernatremia relate to brain cell shrinking. Because too rapid correction of hypernatremia can lead to cerebral edema and convulsions, correction should be made gradually. Again, with no data to support this stance, we believe that all patients undergoing surgery should have serum sodium concentrations of less than 150 mEq/L before anesthesia.
Hypokalemia and hyperkalemia are also discussed in Chapter 25 , Chapter 37 , Chapter 41 , and Chapter 46 . The relationship between the measured potassium concentration in serum and total-body potassium stores can best be described with a scattergram.[186] [763] Only 2% of total-body potassium is stored in plasma (4200 mEq in cells and 60 mEq in extracellular fluid). In normal persons, 75% of the 50 to 60 mEq/L of total-body potassium is stored in skeletal muscle, 6% in red blood cells, and 5% in the liver. Thus, a 20% to 25% change in potassium levels in plasma could represent a change in total-body potassium of 1000 mEq or more if the change were chronic or as little as 10 to 20 mEq if the change were acute.
As with serum sodium levels,[761] [762] acute changes in serum potassium levels appear to be less well tolerated
Hyperkalemia can result from factitious elevation of potassium (as in red blood cell hemolysis); excessive exogenous potassium from sources such as salt substitutes or, in large amounts, bananas; cellular shifts in potassium (as a result of metabolic acidosis, tissue and muscle damage after burns, use of depolarizing muscle relaxants, or intense catabolism of protein); and decreased renal excretion (as occurs in renal failure, renal insufficiency with trauma, and therapy with potassium-sparing diuretic drugs, especially when combined with ACE inhibitors or mineralocorticoid deficiency).[763] [764] [765] [766] [767] [768] [769] Factitious hyperkalemia can occur when a tourniquet is left on too long or even by simple fist clenching.[770]
The major danger in anesthetizing patients who have disorders in potassium balance appears to be abnormal cardiac function—that is, both electrical disturbance[764] [765] [766] [767] and poor cardiac contractility.[765] [766] Hyperkalemia lowers the resting membrane potential of excitable cardiac cells and decreases the duration of the myocardial action potential and upstroke velocity. This decreased rate of ventricular depolarization, plus the beginning of repolarization in some areas of the myocardium while other areas are still undergoing depolarization, produces a progressively widening QRS complex that merges with the T wave into a sine wave on the ECG.
Above a potassium level of 6.7 mEq/L, the degree of hyperkalemia and the duration of the QRS complex correlate well.[764] This correlation is even better than the correlation between the serum potassium level and T-wave changes. Nevertheless, the earliest manifestations of hyperkalemia are narrowing and peaking of the T wave. Though not diagnostic of hyperkalemia, T waves are almost invariably peaked and narrow when serum potassium levels are 7 to 9 mEq/L. When serum potassium levels exceed 7 mEq/L, atrial conduction disturbances appear, as manifested by a decrease in P-wave amplitude and an increase in the PR interval. Supraventricular tachycardia, atrial fibrillation, PVCs, ventricular tachycardia, ventricular fibrillation, or sinus arrest may all occur.
The ECG and cardiac alterations associated with hyperkalemia are potentiated by low serum levels of calcium and sodium. Intravenous administration of saline, bicarbonate, glucose with insulin (1 U/2 g glucose), and calcium can reverse these changes by shifting some extracellular potassium into the cell.
β-Adrenergic stimuli also cause redistribution of potassium into the cell. Indeed, the plasma potassium concentration measured in samples immediately before surgery is usually 0.2 to 0.8 mEq/L lower than that measured during the less stressful period 1 to 3 days before surgery.[771] β-Adrenergic receptor blocking drugs such as propranolol can be used to prevent such an effect preoperatively. A β-adrenergic receptor stimulating agent (20 mg of nebulized albuterol for a 70-kg patient) can be used to treat hyperkalemia when it occurs; it decreases potassium levels 1.0 mEq/L within 30 minutes, and its effect lasts 2 hours.[772] Although nebulized β2 -agonists effectively lower plasma potassium concentrations by stimulating sodium- and potassium-dependent adenosine triphosphatase, this therapy should be used as an adjunct to rather than a substitute for more established measures. Kayexalate (sodium polystyrene sulfonate) enemas can be given to bind potassium in the gut in exchange for sodium. Dialysis against a hypokalemic solution will also decrease serum potassium levels. However, in a hyperkalemic patient, hypoventilation can be dangerous during anesthesia[654] [658] [773] [774] because each 0.1 change in pH can produce a 0.4- to 1.5-mEq/L change in serum potassium levels in the opposite direction. For example, if pH decreases from 7.4 to 7.3, serum potassium levels could increase from 5.5 to 6.5 mEq/L.[767]
Hypokalemia can be caused by inadequate intake of potassium, excessive GI loss (through diarrhea, vomiting, nasopharyngeal suctioning, chronic use of laxatives, or ingestion of cation exchange resins, as in certain wines), excessive renal loss (because of use of diuretic drugs, renal tubular acidosis, chronic chloride deficiency, metabolic alkalosis, mineralocorticoid excess, excessive ingestion of licorice, use of antibiotics, ureterosigmoidostomy, and diabetic ketoacidosis), and shifts of potassium from extracellular to intracellular compartments (as occur in alkalosis, insulin administration, β-adrenergic agonist administration or stress, barium poisoning, and periodic paralysis). As with hyperkalemia, knowledge of the cause of potassium deficiency and the appropriate preoperative evaluation and treatment of that cause may be as important as treatment of the deficiency itself. Also like hyperkalemia, hypokalemia may reflect small or vast changes in total-body potassium. Acute hypokalemia may be much less well tolerated than chronic hypokalemia. The major worrisome manifestations of hypokalemia pertain to the circulatory system, both the cardiac and peripheral components. In addition, chronic hypokalemia results in muscle weakness, hypoperistalsis, and nephropathy.
Cardiovascular manifestations of hypokalemia include autonomic neuropathy, which results in orthostatic hypotension and decreased sympathetic reserve; impaired myocardial contractility; and electrical conduction abnormalities, which can result in sinus tachycardia, atrial and ventricular arrhythmias, and disturbances in intraventricular conduction that can progress to ventricular fibrillation. That these are real concerns for a hypokalemic patient has been shown far too often.[764] [765] [766] [772] [775] [776] [777] In addition to arrhythmias, the ECG shows widening of the QRS complex, ST-segment abnormalities, progressive diminution of the T-wave amplitude, and progressive increase in the U-wave amplitude.[778] Surawicz[764] found these changes to be invariably present when serum potassium levels decreased to below 2.3 mEq/L. Although U waves are not specific for hypokalemia, they are sensitive indicators of the condition. Replenishing the total-body potassium deficit for a depletion reflected by a serum deficit of 1 mEq/L (e.g., from 3.3 to 4.3 mEq/L) may require 1000 mEq of potassium. Even if this amount could be given instantaneously (and it should not be replenished at a rate exceeding 250 mEq/day), it would take 24 to 48 hours to equilibrate in all tissues.[187] [188] Potassium-depleted myocardium is unusually sensitive to digoxin, calcium, and most important, potassium.
Thus, the decision to proceed with surgery and anesthesia in the face of acute or chronic depletions or excesses of potassium depends on many factors. [783] [784] [785] [786] [787] [788] [789] [790] One must know the cause and treatment of the underlying condition creating the electrolyte imbalance and the effect of that imbalance on perioperative risk and physiologic processes. The urgency of the operation, the degree of electrolyte abnormality, the medications given, the acidbase balance, and the suddenness or persistence of the electrolyte disturbance are all considerations. For example, one small study of patients undergoing vascular access procedures with preoperative potassium levels of greater than 6 mmol/L demonstrated no adverse outcomes.[788] Similarly, in a cohort study in which 38 patients had a preoperative potassium level over 5.5 mEq/L, there were no dysrhythmias or major morbidity associated with the use of succinylcholine.[789]
Retrospective epidemiologic studies attribute significant risk to the administration of potassium (even chronic oral administration).[784] [785] In one study, 1910 of 16,048 consecutive hospitalized patients were given oral potassium supplements. Of these 1910 patients, hyperkalemia contributed to death in 7, and the incidence of complications of potassium therapy was 1 in 250. Armed with such data, many internists do not prescribe oral potassium therapy for patients given diuretic drugs. Yet these patients frequently become moderately hypokalemic.[791] [792] Modest hypokalemia occurs in 10% to 50% of patients given diuretic drugs. Should surgery be delayed to subject such patients to the risks of potassium therapy?
Three studies investigated whether modest hypokalemia was a problem by prospectively seeking arrhythmias on the ECGs of patients who had various preoperative levels of potassium.[786] [787] [790] No difference in the incidence of arrhythmias occurred in 25 normokalemic (K > 3.4 mEq/L) patients, 25 moderately hypokalemic (K = 3 to 3.4 mEq/L) patients, and 10 severely hypokalemic (K < 2.9 mEq/L) patients. [786] Wahr and coauthors studied 2402 patients undergoing elective coronary artery bypass grafting and reported that a serum potassium level less than 3.5 mmol/L was a predictor of serious perioperative arrhythmia (odds ratio [OR], 2.2; 95% CI, 1.2 to 4.0), intraoperative arrhythmia (OR, 2.0; 95% CI, 1.0 to 3.6), and postoperative atrial fibrillation/flutter (OR, 1.7; 95% CI, 1.0 to 2.7). [790] The inability of the eye to pick up these changes—or even the inability of Holter recordings for short periods[793] (which seem to not have been obtained in this study)—points to the need for confirming studies.
Other studies indicate that modest hypokalemia can have severe consequences.[792] [794] [795] Holland and coworkers[795] treated 21 patients with 50 mg of hydrochlorothiazide twice a day for 4 weeks. These patients had a history of becoming hypokalemic during diuretic therapy; none of them had cardiac disease or was taking other medication. Before and after diuretic therapy, 24-hour ambulatory ECGs were recorded. This study is also subject to the limitations of Holter monitoring.[793] Ventricular ectopy, including complex ventricular ectopy (multifocal PVCs, ventricular couplets, ventricular tachycardia), developed in 7 of the 21 patients (33%). Potassium repletion decreased the number of etopic ventricular beats per patient from 71.2 to 5.4/hr. Apparently, some patients are sensitive to even minor potassium depletion. In the Multiple Risk Factor Intervention Trial involving 361,662 patients, more than 2000 of whom were treated for hypertension with diuretics, the reduction in serum potassium after diuretic therapy was greater in those with PVCs.[792] Although we recommend that hypokalemic patients be given potassium supplements, the merit of this practice is unclear.
Our personal criteria for preoperative potassium therapy are as follows. As a rule, all patients undergoing elective surgery should have normal serum potassium levels. However, we do not recommend delaying surgery if the serum potassium level is above 2.8 mEq/L or below 5.9 mEq/L, if the cause of the potassium imbalance is known, and if the patient is in otherwise optimal condition. This range of safe potassium levels is arbitrary and has changed from 3.3 in 1979, to 3.1 in 1986, to 2.9 in 1990 on the lower side and from 5.6 in 1979, to 5.7 in 1986, to 5.9 in 1990 on the upper side as more data have become available on the safety of preoperative hypokalemia and the dangers of replacing potassium in a hospital environment[784] [785] (see Table 25-5 in Chapter 25 ). We subject all patients with end-stage renal failure to dialysis (using the same arbitrary safe range) before all surgical procedures except truly emergency ones (as in instances of imminent exsanguination). In studies on dogs in 1978, Tanifuji and Eger[796] determined the relationships between electrolyte status and anesthetic requirements that may require intraoperative consideration: hyponatremia and hypoosmolality decreased the minimum alveolar concentration (MAC), hypernatremia increased MAC, and hyperkalemia did not affect the anesthetic requirement.
|
|
|
|
|
|
|
|
|
|
|
|
|