|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
One may ask why preoperative preparation of a patient with renal disease is discussed in the same section as preoperative preparation of a patient with an infectious disease. Although it is commonly recommended that no surgery except emergency or curative (e.g., drainage of an abscess) operations be performed in patients with infectious disease, it has become evident that renal insufficiency can be caused by antimicrobial drugs[684] and that sepsis, not shock, is probably the leading cause of acute postoperative renal failure. [685] The anesthesiologist has an important role to play in preventing the onset and consequences of renal failure and its initiators. [686] [687] The linking of renal failure to electrolyte disorders is more obvious: the kidney is the primary organ for regulating body osmolality and fluid volume and has a major role in excretion of the end products of metabolism. In performing these functions, the kidney becomes intimately involved in the excretion of electrolytes.
A patient with renal insufficiency whose own kidneys are still functioning is distinct not only from a patient with end-stage renal disease whose renal functions are provided by dialysis but also from a patient who has a transplanted kidney. These three groups of patients require very different preoperative preparation. In addition, acute changes in renal function present quite a different problem than do chronic alterations in function.[686] [687] Certain renal diseases require different preoperative preparation than others, but generally, renal disease of any origin presents the same preoperative problems (also see Chapter 20 , Chapter 37 , and Chapter 54 ).
Nephrotic syndrome may develop in patients with glomerular diseases without disturbing tubular function. The soundness of tubular function is an important consideration because tubular dysfunction with attendant uremia presents quite different problems than glomerular disease with only nephrotic syndrome does. This is not to minimize the adverse effects of glomerular disease; nephrotic syndrome consists of massive proteinuria and consequent hypoalbuminemia. The resulting reduction in plasma oncotic pressure diminishes plasma volume and calls forth compensatory mechanisms that result in retention of sodium and water. As a result, a common clinical finding in nephrotic syndrome is edema. Thus, patients with nephrotic syndrome may have excess total-body water and decreased intravascular volume. In addition, diuretics are often given in an attempt to decrease edema. Although serum creatinine and creatinine clearance have limitations as indices of the glomerular filtration rate (GFR) (inulin clearance is still the gold standard), these measurements are, for now, the most readily available to the anesthesiologist.[687] Plasma creatinine levels reflect endogenous muscle catabolism and dietary intake, as well as urinary excretion. Urinary excretion depends on both filtration and secretion by the kidney. Drugs that are commonly used in the preoperative and perioperative periods can distort this measure of glomerular filtration: cimetidine and trimethoprim interfere with secretion by increasing plasma creatinine and decreasing creatinine clearance without altering filtration.[687] It should also be remembered that the commonly used methods for measuring creatinine have a 95% confidence limit of more than 20% for a GFR greater than 30 mL/min. Thus, a normal creatinine level of 1.3 mg/dL might give a measured value ranging from 1.0 to 1.5 mg/dL.[687]
Furthermore, in patients with nephrotic syndrome in whom renal tubular function has been preserved, hypovolemia appears to be a significant cause of deteriorating tubular renal function.[687] [688] [689] [690] [691] Consequently, we advocate the same intense preoperative, intraoperative, and post-operative fluid management for patients with nephrotic syndrome as we do for patients with diminished tubular function. Admittedly, no randomized study has shown that close control of intravascular volume status in these groups of patients preserves renal tubular function (or any other measure of perioperative morbidity) to a greater degree than less rigid control does.
Uremia, the end result of renal tubular failure (i.e., failure of the concentrating, diluting, acidifying, and filtering functions), is manifested in many ways. Changes occur in the cardiovascular, immunologic, hematologic, neuromuscular, pulmonary, and endocrine systems, as well as in bone. These alterations are ascribed either to the toxic end products of protein metabolism or to an imbalance in functioning of the kidney. As the number of functioning nephrons diminishes, the still-functioning nephrons attempt to increase some solute and body composition preservation functions at the expense of other functions, such as excretion of phosphate. The accumulation of phosphate increases parathormone levels, which in turn produces osteodystrophy. Osteodystrophy can be managed by (1) restriction of dietary phosphate, (2) the use of gels (e.g., aluminum hydroxide or carbonate) that bind with intestinal phosphate, (3) calcium supplementation, or (4) parathyroidectomy.[692]
Certain alterations in patients with uremia, such as neuropathy, are most logically attributed to an accumulation of toxic metabolites. Peripheral neuropathy is most often sensory and involves the lower extremities, but it may also be motor; peripheral neuropathies are often
Cardiac failure (especially episodic failure) frequently occurs in uremic patients because of the presence of many adverse conditions: anemia with increasing myocardial work, hypertension, atherosclerosis, and altered volume status. Pericarditis can manifested by pericardial rub alone or by pain (with or without hemorrhage). Cardiac tamponade should be ruled out on the basis of clinical features and by echocardiography if this diagnosis is seriously suspected preoperatively. In addition, cardiac tamponade should be treated or planned for preoperatively.
If anemia is present, its severity generally parallels the degree of uremia; chronically uremic patients seem to adapt well to anemia. No hard data have substantiated the need to give a preoperative blood transfusion to a chronically uremic patient, even when the preoperative hematocrit is as low as 16 or 18 vol%. Even in nonuremic patients in an ICU, a recent randomized trial was unable to demonstrate improved outcome with a liberal transfusion strategy.[695] One of the major historical reasons for not transfusing blood in patients with end-stage renal disease has been disproved: data show that the more blood transfusions a transplant recipient receives before transplantation, the greater the chance that the transplant will function successfully.[696] This immunosuppressive effect of transfusions is now routinely used in transplantation. However, the development of recombinant human erythropoietin may obviate the need for transfusions in chronically uremic patients in the future. Administration of recombinant human erythropoietin to patients in chronic and progressive renal failure resulted in an average increase in hematocrit of 10% above baseline within 3 weeks, with no serious side effects. [697] Balancing of the immunosuppressive use of blood transfusions versus the benefits of erythropoietin and the risks of transfusion remains to be determined. In uremic patients, coagulation and platelet adhesiveness may be abnormal and factor III activity decreased. Even uremic patients not given corticosteroids or immunosuppressive drugs may demonstrate abnormal immunity, perhaps meriting increased attention regarding procedures that lessen patient cross-contamination.
Uremic patients exhibit a wide variety of metabolic and endocrinologic disorders in addition to hyperparathyroidism,[692] including impaired carbohydrate tolerance, insulin resistance, type IV hyperlipoproteinemia, autonomic insufficiency, hyperkalemia, and anion-gap acidosis (caused by an inability of the kidneys to reabsorb filtered bicarbonate and excrete sufficient ammonium into urine).[698] Furthermore, the excretion and pharmacokinetics of drugs are different in uremic patients than in normal patients. In addition, complications of hemodialysis include hepatitis B (and persistent hepatitis B antigenemia), nutritional deficiencies, electrolyte and fluid imbalances, and mental disorders. Because these conditions can lead to serious perioperative morbidity, they should be evaluated before surgery. No data, however, have substantiated the hypothesis that preoperative optimization of these metabolic and endocrinologic disorders reduces perioperative risk in uremic patients.
As with uremic patients, preoperative optimization of volume status is paramount in patients with kidney stones.[699] Seventy-five percent of all kidney stones are composed of calcium oxalate. Patients with these stones often take diuretic drugs, avoid calcium-rich foods, or restrict salt intake. Prevention of dehydration by institution of intravenous fluid therapy along with restricted oral intake of protein may be as important for these patients as it is for patients with struvite or uric acid stones. Struvite stones often result from urinary infection. Uric acid stones can be prevented by treatment with allopurinol, by preoperative hydration, or by alkalization of urine. Acidosis may contribute to stone formation. Again, optimal intravascular volume status is important in preventing stones and preserving renal function. More thorough discussion of renal function and physiology is provided in Chapter 20 . Chapter 54 deals with the complexities of managing patients for renal surgery and other urologic procedures.
Creatinine clearance in conjunction with free water clearance
appears to be the most accurate way of quantifying, for pharmacokinetic purposes,
the degree of decreased renal function[700]
[701]
(also see Chapter 37
). For
a patient with stable renal function, creatinine clearance, which is a rough estimate
of GFR, can be approximated by noting the serum creatinine level: a doubling of
the creatinine level represents a halving of GFR. Thus, a patient with a stable
serum creatinine level of 2 mg/dL would have a GFR of approximately 60 mL/min. A
stable serum creatinine level of 4 mg/dL would accompany a GFR of approximately 30
mL/min, and a stable serum creatinine level of 8 mg/dL would accompany a GFR of 15
mL/min or less. When pregnancy and considerable edema are not present and the serum
creatinine level is stable, the following formulas can be used to estimate creatinine
clearance and free water clearance.[700]
[702]
[703]
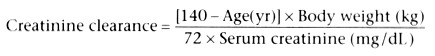

Note that renal function must be stable. Unstable renal function is often associated
with changes in serum creatinine levels that lag by several days. Although knowing
Free water clearance is a measure of renal concentrating ability and is normally -25 to +100 mL/hr; it becomes more positive in renal insufficiency states. It may also become more positive in patients who have a head injury or high blood alcohol levels or in those undergoing aggressive fluid infusion or administration of diuretics.[701]
One of the greatest challenges for the anesthesiologist is presented by patients with insufficient renal function whose renal function must be preserved during surgery. Additionally, the presence of chronic renal failure is associated with higher rates of perioperative cardiac morbidity, which may warrant further evaluation for the presence of occult coronary artery disease.[704] The many uremic symptoms and great perioperative morbidity associated with uremia can probably be avoided by attention to detail in the preoperative and perioperative management of patients with insufficient, but still functioning kidneys.[685] [686] [687] [688] [689] [690] [691] [692]
First, studies demonstrate that acute postoperative renal failure is associated with an extremely high mortality rate.[399] [400] [401] [403] [410] [705] There are multiple risk factors for the development of perioperative renal dysfunction. The most important risk factors include preexisting renal disease, heart surgery involving cardiopulmonary bypass or aortic surgery involving cross-clamping of the thoracic or abdominal aorta, and ongoing sepsis. In a systematic review of 28 studies by Novis and colleagues, preoperative renal risk factors such as increased serum creatinine, increased BUN, and preoperative renal dysfunction were repeatedly found to predict postoperative renal dysfunction.[702] Mangano and coworkers performed a prospective cohort study of 2222 patients with or without concurrent vascular surgery.[705] They identified five independent preoperative predictors of renal dysfunction: age 70 to 79 years (relative risk [RR], 1.6; 95% CI, 1.1 to 2.3) or age 80 to 95 years (RR, 3.5; CI, 1.9 to 6.3), CHF (RR, 1.8; CI, 1.3 to 2.6), previous myocardial revascularization (RR, 1.8; CI, 1.2 to 2.7), type 1 diabetes mellitus (RR, 1.8; CI, 1.1 to 3.0) or preoperative serum glucose levels exceeding 16.6 mmol/L (RR, 3.7; CI, 1.7 to 7.8), and preoperative serum creatinine levels of 124 to 177 µmol/L (RR, 2.3; CI, 1.6 to 3.4).
Moreover, acute perioperative renal failure is most likely to occur in patients who have renal insufficiency before surgery, are older than 60 years, and have preoperative left ventricular dysfunction.[706] Proper hydration before surgery probably decreases the mortality after acute renal failure induced by radiocontrast agents.[690] [691] [692] Clues regarding the presence of hypovolemia or hypervolemia should be sought from the history and physical examination (e.g., weight loss or gain, thirst, edema, orthostatic hypotension and tachycardia, flat neck veins, dry mucous membranes, decreased skin turgor). In seriously ill patients, insertion of a pulmonary arterial catheter will permit more precise monitoring of intravascular fluid volume. Other causes of deterioration in function in chronic renal insufficiency are low cardiac output or low renal blood flow (in prerenal azotemia, whether because of cardiac failure or because of fluid depletion from diuretic drugs, BUN often increases disproportionately to increases in creatinine), urinary tract infection, use of nephrotoxic drugs, hypercalcemia, and hyperuricemia. These conditions and drugs should be avoided; if any of these conditions exist, they should be treated preoperatively.
To preserve normal renal function, infusion of saline, mannitol, furosemide, or low-dose dopamine has been recommended.[707] [708] [709] [710] [711] [712] [713] However, these therapies should be initiated with caution because saline infusions and mannitol can lead to fluid overload and myocardial damage; in addition, diuretic drugs given intraoperatively can produce postoperative hypovolemia, which worsens renal function. Very high concentrations of mannitol may also cause acute renal failure.[714] A meta-analysis of 58 trials involving the use of dopamine for renal protection/treatment, only 17 of which were randomized, was unable to demonstrate any benefit from the use of low-dose dopamine.[715] Maintenance of normal intravascular fluid volume can be guided by pulmonary capillary wedge pressure and has prevented impairment of renal function after abdominal aortic reconstruction, even when urinary volumes were low.[716] Fenoldopam is a dopamine analog that causes natriuresis and increases renal blood flow and urine output.[717] Several small studies have reported renoprotective effects of fenoldopam in patients undergoing cardiopulmonary bypass and infrarenal aortic aneurysm repair.[718] [719] [720]
Anesthetic drugs have been studied in patients with renal insufficiency. In a randomized trial, general anesthesia with desflurane or isoflurane did not exacerbate renal insufficiency.[721] Despite concern that sevoflurane leads to the production of compound A, which impairs renal functioning, a randomized trial of low-flow sevoflurane versus low-flow isoflurane anesthesia in patients with stable renal insufficiency did not demonstrate any difference in any measured renal function parameter.[722]
Patients with chronic (and at times acute) renal failure require renal replacement therapy, including conventional intermittent hemodialysis, peritoneal dialysis, and continuous renal replacement therapy (CRRT). CRRT includes a wide variety of techniques whose perioperative management has recently been reviewed[723] ( Table 27-47 ). Although the primary indication for CRRT is acute renal failure, it can also be used for fluid clearance, correction of electrolyte abnormalities, and management of metabolic acidosis. It can be used in surgical patients without significant hemodynamic abnormalities. These patients may return to the operating room and their assessment and management may be complicated by the underlying disease and the use of systemic anticoagulation to prevent filter and circuit clotting. In patients undergoing intermittent treatment with hemodialysis or peritoneal dialysis, the procedure is discontinued before entering the operating room. For CRRT, the anesthesiologist must determine the appropriateness of discontinuing the therapy. With short procedures, the therapy can almost always be stopped and the arterial and venous ends of the circuit connected and run in the bypass mode. CRRT can also be used to manage fluids during surgery by changing the dialysate. If CRRT is continued, its effect on drug dosing
| Renal Replacement Therapy | Blood Pump | Replacement Fluid (RF)/Dialysate (D) | Intraoperative Use |
|---|---|---|---|
| Conventional intermittent hemodialysis | Yes | D | No |
| Peritoneal dialysis | No | D | No |
| Slow continuous ultrafiltration | Yes/no | None | Yes |
| Continuous arteriovenous hemodialysis | No | D | No |
| Continuous arteriovenous hemodiafiltration | No | RF/D | No |
| Continuous venovenous hemofiltration | Yes | RF | Yes |
| Continuous venovenous hemodialysis | Yes | D | Yes |
| Continuous venovenous hemodiafiltration | Yes | RF/D | Yes |
| From Petroni KC, Cohen NH: Continuous renal replacement therapy: Anesthetic implications. Anesth Analg 94:1288–1297, 2002, with permission. | |||
Because a patient undergoing dialysis has already lost natural renal functioning, the emphasis in preoperative assessment shifts toward protecting other organ systems and optimally maintaining vascular access sites for cannulation. Usually, this does not require invasive monitoring. Emphasis is placed on intravascular fluid volume and electrolyte status, which can be ascertained by knowing when the patient last underwent dialysis, how much weight was normally gained or lost with dialysis, whether the fluid loss was peritoneal or intravascular, and what electrolyte composition the blood was dialyzed against. Although preoperative dialysis may benefit patients who have hyperkalemia, hypercalcemia, acidosis, neuropathy, and fluid overload, the resulting dysequilibrium between fluid and electrolytes can cause problems. Because hypovolemia induced by dialysis can lead to intraoperative hypotension, we try to avoid weight and fluid reduction in patients undergoing preoperative dialysis. In addition, hypopnea has been found to occur during and after dialysis when the dialysate contained acetate.[724] Avoiding an acetate bathing solution may prevent this cause of hypoventilation.
More than 140,000 patients have received renal transplants (versus 300,000 currently undergoing dialysis in the United States). Approximately 60% are still alive, although a third must undergo dialysis (approximately 50,000 patients are now awaiting transplantation).[725] The advantages, in addition to increased survival, include improvement of the anemia, endocrine and sexual function, and fatigue that inhibit employment and enjoyment for dialysis patients. The disadvantages are the cost and risks of the transplant operation and the chronic use of immunosuppressants. When these patients have subsequent surgery, the status of their renal function must be determined (i.e., whether they have normal renal function, insufficient but still functioning kidneys, or end-stage renal disease requiring hemodialysis). Descriptions of side effects from immunosuppressive drugs should also be sought. The drugs used preoperatively and intraoperatively to prevent acute rejection themselves have serious side effects that encourage close monitoring of blood glucose and cardiovascular function.[696] [726] [727] Because renal transplantation greatly increases the risk of infection, it is very important to avoid invasive monitoring and prevent patient cross-contamination.
Patients with renal azotemia have a threefold or higher risk of an adverse drug reaction than those with normal renal function do.[728] [729] [730] [731] [732] [733] The risk is increased by two conditions. (1) Excessive pharmacologic effects result from high levels of a drug or its metabolite (e.g., the metabolite of meperidine) in blood because of physiologic changes in target tissues induced by the uremic state. An example would be excessive sedation in a uremic patient with standard blood levels of sedative-hypnotic drugs. (2) Excessive administration of electrolytes with drugs also increases the risk of an adverse drug reaction. For example, penicillin standardly has 1.7 mEq of potassium per 1 million U.[728] [729] [730] [731] [732] [733] Administration of standard doses of drugs that depend on renal excretion for their elimination can result in drug accumulation and enhanced pharmacologic effect. Bennett and associates[728] and Gibson[731] have provided dosing guidelines for many drugs used by anesthesiologists for patients with and without renal failure. For example, patients with end-stage renal disease required significantly higher propofol doses to achieve the clinical end point of hypnosis than patients with normal renal function did.[732]
|
|
|
|
|
|
|
|
|
|
|
|
|